Biolight
GUDID 00852503007136
Biolight 15.1" multi-parameter modular patient monitor
VENNI INSTRUMENTS INC.
Single-patient intensive/general healthcare physiologic monitoring system| Primary Device ID | 00852503007136 |
| NIH Device Record Key | 5407ca91-f1fe-4944-9ee7-b8f3bff21bc9 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Biolight |
| Version Model Number | Q7 |
| Company DUNS | 960516735 |
| Company Name | VENNI INSTRUMENTS INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00852503007136 [Primary] |
FDA Product Code
| MWI | Monitor,Physiological,Patient(Without Arrhythmia Detection Or Alarms) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2020-02-06 |
| Device Publish Date | 2016-10-06 |
On-Brand Devices [Biolight]
| 00852503007150 | Biolight Vital Signs Monitor |
| 00852503007143 | Biolight 7" multi-parameter patient monitor |
| 00852503007136 | Biolight 15.1" multi-parameter modular patient monitor |
| 00852503007129 | Biolight 12.1" multi-parameter modular patient monitor |
| 00852503007112 | Biolight 15.1" multi-parameter patient monitor |
| 00852503007105 | Biolight 12.1" multi-parameter patient monitor |
| 00852503007099 | Biolight 10.4" multi-parameter patient monitor |
| 00852503007082 | Biolight 8" multi-parameter patient monitor |
Trademark Results [Biolight]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
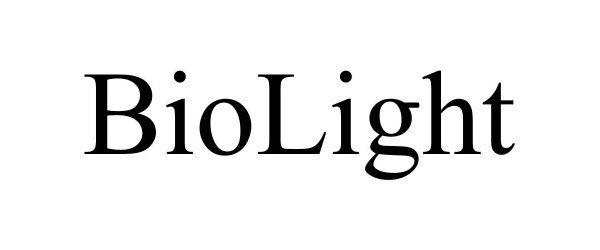 BIOLIGHT 97867724 not registered Live/Pending |
3D Systems, Inc. 2023-03-31 |
 BIOLIGHT 90687742 not registered Live/Pending |
Aqua Science LLC 2021-05-03 |
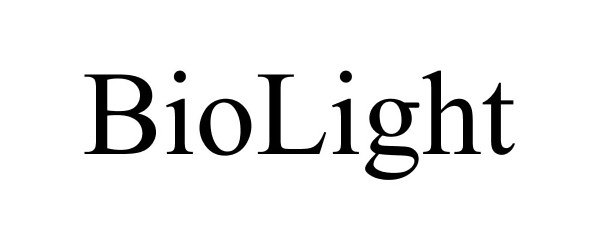 BIOLIGHT 88457474 not registered Live/Pending |
Biolight 2019-06-03 |
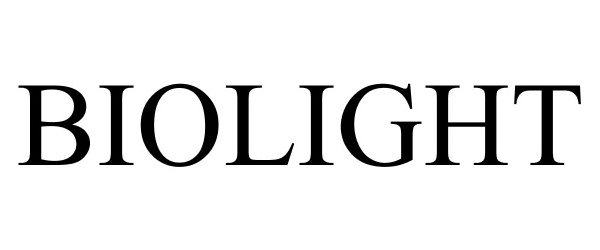 BIOLIGHT 87552669 5412530 Live/Registered |
Sarkli-Repechage, Ltd. 2017-08-02 |
 BIOLIGHT 87532623 not registered Dead/Abandoned |
Jose Fernandez 2017-07-18 |
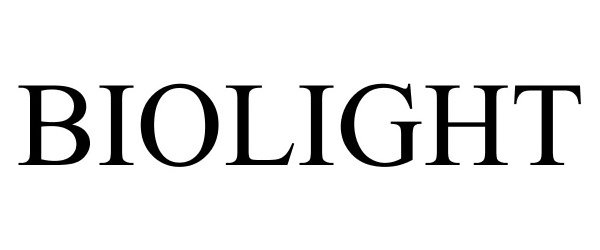 BIOLIGHT 86127920 4694274 Live/Registered |
Synca Marketing Inc 2013-11-25 |
 BIOLIGHT 85811166 4379964 Live/Registered |
Bioscan, Inc. 2012-12-27 |
 BIOLIGHT 85305267 not registered Dead/Abandoned |
Sarkli-Repechage, Ltd. 2011-04-26 |
 BIOLIGHT 77533039 not registered Dead/Abandoned |
Biolight Harvesting, Inc. 2008-07-28 |
 BIOLIGHT 77397367 not registered Dead/Abandoned |
Cyalume Technologies, Inc. 2008-02-14 |
 BIOLIGHT 77325722 not registered Dead/Abandoned |
MARQUEZ BROTHERS INTERNATIONAL, INC. 2007-11-09 |
 BIOLIGHT 75457943 not registered Dead/Abandoned |
BRYAN, BRUCE 1998-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.