FFRct
GUDID 00853341006015
HeartFlow FFRct is an image analysis software developed for the clinical quantitative and qualitative analysis of previously acquired Computed Tomography DICOM data. It is a tool for the analysis of CT DICOM-compliant cardiac images and data, to assess the anatomy and function of the coronary arteries. The software displays the anatomy combined with functional information using graphics and text, including computed and derived quantities of blood flow, pressure and velocity, to aid the clinician in the assessment of coronary artery disease. HeartFlow FFRct is independent of imaging equipment, imaging protocols and equipment vendors. HeartFlow analyses are performed on previously physician-acquired image data and are unrelated to acquisition equipment and workstations.
HEARTFLOW, INC.
Vascular modelling web-based application software| Primary Device ID | 00853341006015 |
| NIH Device Record Key | 10f27d5e-3a6b-4743-abdf-03e23643ea7d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FFRct |
| Version Model Number | v2.1 |
| Company DUNS | 804411283 |
| Company Name | HEARTFLOW, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com | |
| Phone | +16502410500 |
| support@heartflow.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00853341006015 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K161772
FDA Product Code
| PJA | Coronary Vascular Physiologic Simulation Software |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-12-06 |
On-Brand Devices [FFRct]
| 00853341006022 | HeartFlow FFRct is coronary physiologic simulation software developed for the clinical quantitat |
| 00853341006015 | HeartFlow FFRct is an image analysis software developed for the clinical quantitative and qualit |
| 00853341006008 | HeartFlow FFRct is an image analysis software developed for the clinical quantitative and qualit |
Trademark Results [FFRct]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
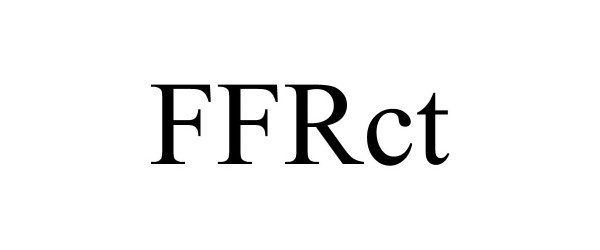 FFRCT 90808627 not registered Live/Pending |
Elucid Bioimaging Inc. 2021-07-02 |
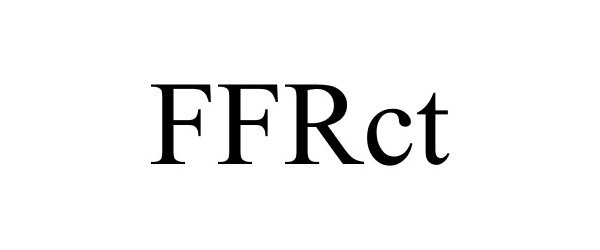 FFRCT 85334348 not registered Dead/Abandoned |
Heartflow, Inc. 2011-05-31 |
 FFRCT 85284255 4641484 Dead/Cancelled |
Heartflow, Inc. 2011-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.