PALS
GUDID 00856817005499
AXELGAARD MANUFACTURING CO., LTD.
Transcutaneous electrical stimulation electrode| Primary Device ID | 00856817005499 |
| NIH Device Record Key | a85f62df-f13d-4672-9e6f-7fc3dc8ab135 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | PALS |
| Version Model Number | 895250 |
| Company DUNS | 150959401 |
| Company Name | AXELGAARD MANUFACTURING CO., LTD. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com | |
| Phone | 760-451-8000 |
| support@axelgaard.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
| Storage Environment Temperature | Between 41 Degrees Fahrenheit and 80.6 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00856817005499 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K132422
FDA Product Code
| GXY | Electrode, Cutaneous |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-03-31 |
On-Brand Devices [PALS]
| 00856817005550 | SC100 |
| 00856817005543 | PR100 |
| 00856817005536 | 896350 |
| 00856817005529 | 896240 |
| 00856817005512 | 896230 |
| 00856817005505 | 895340 |
| 00856817005499 | 895250 |
| 00856817005482 | 895402 |
| 00856817005475 | 895240 |
| 00856817005468 | 895220 |
| 00856817005451 | 891200 |
| 00856817005444 | 891100 |
| 00856817005437 | 879300 |
| 00856817005420 | 879200 |
| 00856817005413 | 879100 |
| 00856817005406 | J10R00 |
| 00851913006265 | COM200 |
Trademark Results [PALS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PALS 98744920 not registered Live/Pending |
Kingsley, Lisa 2024-09-11 |
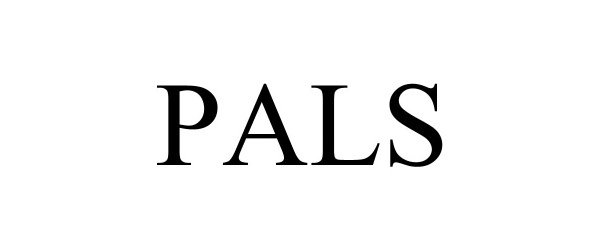 PALS 98440228 not registered Live/Pending |
Pals Health LLC 2024-03-08 |
 PALS 98435801 not registered Live/Pending |
PLEXSYS Interface Products, Inc. 2024-03-06 |
 PALS 98310906 not registered Live/Pending |
Baggerson,Robert H 2023-12-12 |
 PALS 97396436 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396432 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396430 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396429 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396427 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396423 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97396417 not registered Live/Pending |
MASTER OF CEREMONIES LIMITED 2022-05-05 |
 PALS 97395691 not registered Live/Pending |
Urban Resource Institute 2022-05-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.