T-SPeC 114
GUDID 00856995003010
Transurethral Suprapubic endo-Cystostomy
SWAN VALLEY MEDICAL, INCORPORATED
Suprapubic drainage catheter| Primary Device ID | 00856995003010 |
| NIH Device Record Key | 591d7a18-0c9c-4b89-8f53-8acc75579f89 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | T-SPeC |
| Version Model Number | T14 |
| Catalog Number | 114 |
| Company DUNS | 795608012 |
| Company Name | SWAN VALLEY MEDICAL, INCORPORATED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com | |
| Phone | 406-837-1500 |
| cs@swanvalleymedical.com |
Device Dimensions
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
| Catheter Gauge | 18 French |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00856995003010 [Primary] |
| GS1 | 08569950031141 [Previous] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K120127
FDA Product Code
| KOB | Catheter, Suprapubic (And Accessories) |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2020-04-01 |
| Device Publish Date | 2020-03-10 |
On-Brand Devices [T-SPeC]
| 00856995003010 | Transurethral Suprapubic endo-Cystostomy |
| 00856995003003 | Transurethral Suprapubic endo-Cystostomy |
| 08569950031141 | Transurethral Suprapubic endo-Cystostomy |
| 08569950031073 | Transurethral Suprapubic endo-Cystostomy |
Trademark Results [T-SPeC]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
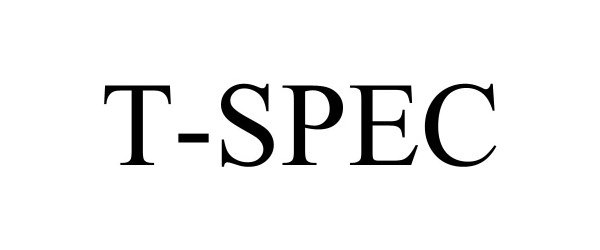 T-SPEC 98222761 not registered Live/Pending |
Swan Valley Medical, Inc. 2023-10-13 |
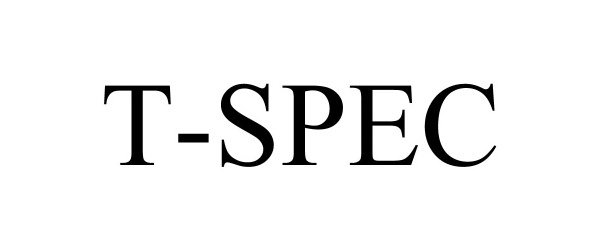 T-SPEC 88861163 not registered Live/Pending |
Nissan Jidosha Kabushiki Kaisha 2020-04-06 |
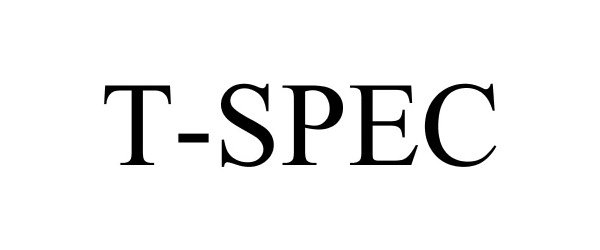 T-SPEC 85672663 4292325 Live/Registered |
Swan Valley Medical, Incorporated 2012-07-10 |
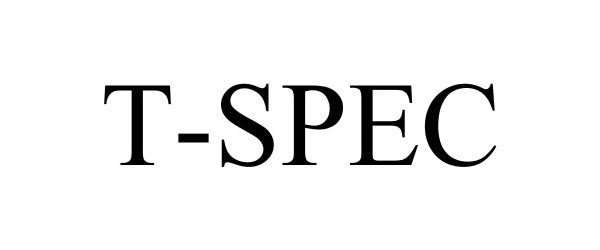 T-SPEC 77433197 4171930 Live/Registered |
Swan Valley Medical, Incorporated 2008-03-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.