DigiView
GUDID 00857050006038
Doctor's Reading Station
Digisonics Inc
Radiology PACS software| Primary Device ID | 00857050006038 |
| NIH Device Record Key | cfdb281c-dd72-408f-a4d5-07541bf05b72 |
| Commercial Distribution Discontinuation | 2021-04-12 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | DigiView |
| Version Model Number | 4.0 |
| Company DUNS | 099807927 |
| Company Name | Digisonics Inc |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00857050006038 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K970402
FDA Product Code
| LLZ | System, Image Processing, Radiological |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-04-20 |
| Device Publish Date | 2021-04-12 |
On-Brand Devices [DigiView]
| 00857050006007 | Doctors Review Station - System, Image processing, Radiological |
| 00857050006038 | Doctor's Reading Station |
Trademark Results [DigiView]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
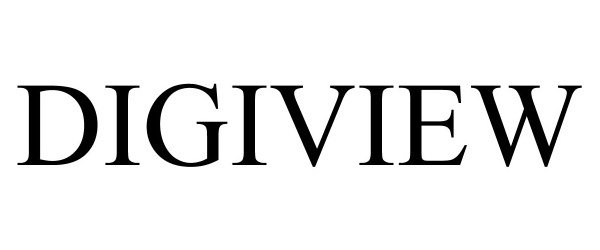 DIGIVIEW 90657846 not registered Live/Pending |
KUB TECHNOLOGIES, INC 2021-04-20 |
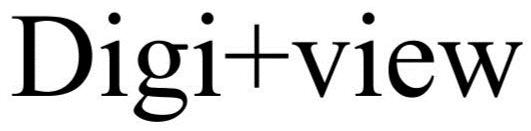 DIGIVIEW 86045011 4496513 Live/Registered |
Diginonymous LLC 2013-08-22 |
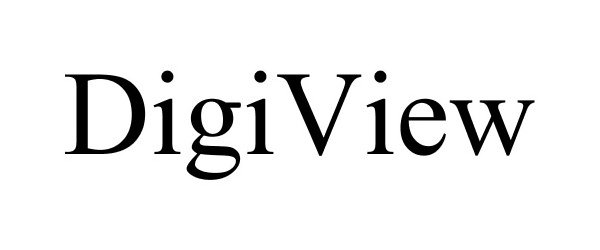 DIGIVIEW 85461188 not registered Dead/Abandoned |
VersEye Inc. 2011-11-01 |
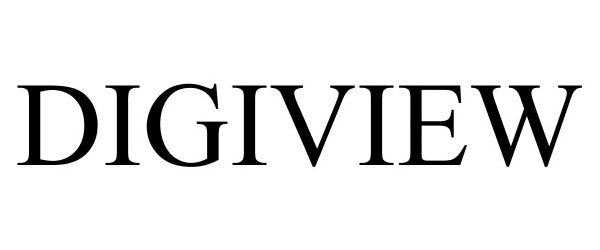 DIGIVIEW 85112060 3965132 Live/Registered |
KUB Technologies, Inc. 2010-08-20 |
 DIGIVIEW 78541983 3105194 Dead/Cancelled |
Hideki Electronics, Inc. 2005-01-04 |
 DIGIVIEW 78114221 not registered Dead/Abandoned |
Continental Technology International, LLC 2002-03-12 |
 DIGIVIEW 77248974 not registered Dead/Abandoned |
VersEye, Inc. 2007-08-07 |
 DIGIVIEW 76601655 not registered Dead/Abandoned |
GKB CCTV CO., LTD. 2004-07-12 |
 DIGIVIEW 76587133 3093655 Dead/Cancelled |
DIGIVIEW PRODUCTIONS, L.L.C. 2004-04-16 |
 DIGIVIEW 76524971 3025073 Live/Registered |
SleepMed, incorporated 2003-06-23 |
 DIGIVIEW 76042636 2524426 Live/Registered |
COBRA ELECTRONICS CORPORATION 2000-05-05 |
 DIGIVIEW 75547880 2488124 Dead/Cancelled |
ED-X, L.L.C. 1998-09-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.