Micro-X K100-0105
GUDID 00858293006120
Micro-X Peracetic Acid Test Strips - Potency
REPROCESSING PRODUCTS CORPORATION
Peracetic acid device disinfectant/sterilant test strip| Primary Device ID | 00858293006120 |
| NIH Device Record Key | ae86e38b-38dd-4ac1-8fbe-e216cbec0203 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Micro-X |
| Version Model Number | K100-0105 |
| Catalog Number | K100-0105 |
| Company DUNS | 796083392 |
| Company Name | REPROCESSING PRODUCTS CORPORATION |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00858293006120 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K974426
FDA Product Code
| LIF | Dialyzer Reprocessing System |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2017-03-07 |
On-Brand Devices [Micro-X]
| 10858293006332 | Micro-X Dialyzer Reprocessing Concentrate |
| 00858293006120 | Micro-X Peracetic Acid Test Strips - Potency |
| 00858293006113 | Micro-X Residual Test Strips |
| 00858293006922 | Micro-X Disinfectant/Sterilant |
Trademark Results [Micro-X]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MICRO-X 78426899 3004079 Dead/Cancelled |
Reprocessing Products Corp 2004-05-28 |
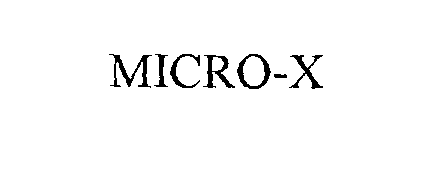 MICRO-X 76207283 not registered Dead/Abandoned |
CARL-ZEISS-STIFTUNG 2001-02-09 |
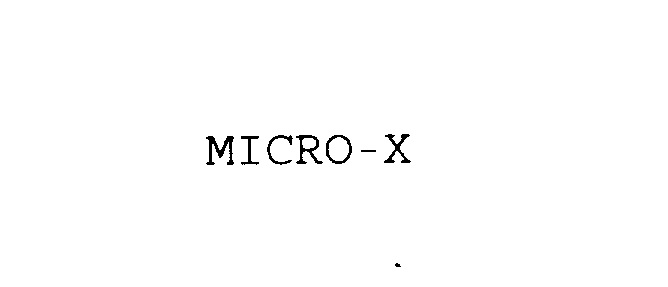 MICRO-X 75860717 2478593 Dead/Cancelled |
METRO LINK, INC. 1999-11-30 |
 MICRO-X 75311184 not registered Dead/Abandoned |
MICRO EXPERTS, INC. 1997-06-18 |
 MICRO-X 72368017 0907460 Dead/Expired |
ALARMTRONICS ENGINEERING, INC. 1970-08-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.