LITEX
GUDID 00858466005172
Curing Light
DENTAMERICA, INC.
Dental/surgical polymerization lamp| Primary Device ID | 00858466005172 |
| NIH Device Record Key | 326171c2-7e44-4348-9834-ad3369e7b6d0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | LITEX |
| Version Model Number | 680A |
| Company DUNS | 174436360 |
| Company Name | DENTAMERICA, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00858466005172 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K955759
FDA Product Code
| EBZ | Activator, Ultraviolet, For Polymerization |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-10-05 |
On-Brand Devices [LITEX]
| 00858466005226 | Curing Light |
| 00858466005202 | Curing Light |
| 00858466005196 | Curing Light |
| 00858466005189 | Curing Light |
| 00858466005172 | Curing Light |
Trademark Results [LITEX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
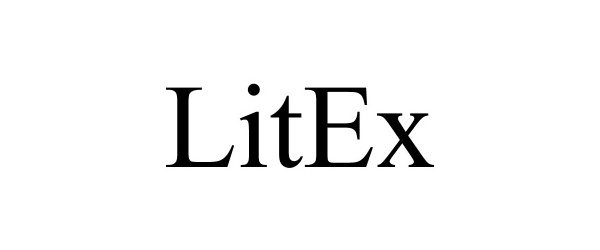 LITEX 97094774 not registered Live/Pending |
Litigation Exchange, LLC 2021-10-27 |
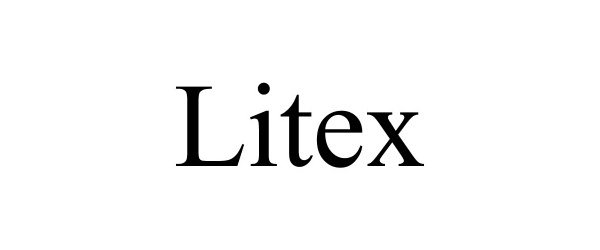 LITEX 90751401 not registered Live/Pending |
Guibin Pan 2021-06-03 |
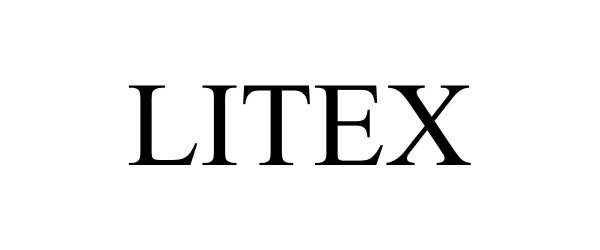 LITEX 85917665 4526945 Live/Registered |
M. Rogers Design, Inc. 2013-04-29 |
 LITEX 81008188 1008188 Dead/Cancelled |
Anglo Fabrics Company, Inc. 0000-00-00 |
 LITEX 79044646 3666859 Live/Registered |
Synthomer Deutschland GmbH 2007-07-26 |
 LITEX 79021654 3291124 Dead/Cancelled |
DESA Tech AG 2006-02-23 |
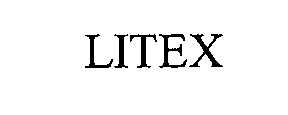 LITEX 76511778 2892727 Live/Registered |
LITEX INDUSTRIES LIMITED 2003-04-17 |
 LITEX 76507471 2892699 Live/Registered |
LITEX INDUSTRIES LIMITED 2003-04-17 |
 LITEX 76507461 not registered Dead/Abandoned |
LITEX INDUSTRIES LIMITED 2003-04-17 |
 LITEX 75862573 2469110 Dead/Cancelled |
K/P Corporation 1999-12-02 |
 LITEX 74686868 2088097 Dead/Cancelled |
AIXTRON SEMICONDUCTOR TECHNOLOGIES GmbH 1995-06-12 |
 LITEX 73796080 1608967 Live/Registered |
HUANG, JERRY T. 1989-04-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.