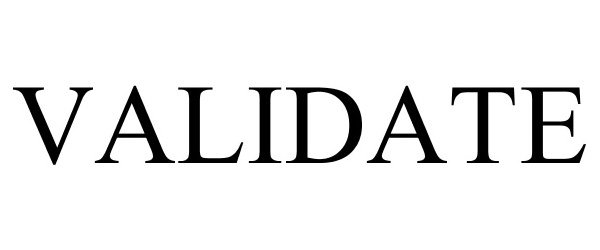| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| Storage Environment Temperature | Between -25 Degrees Celsius and -10 Degrees Celsius |
| 00859110005265 | Validate GC1sv is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005258 | Validate GC1vt is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005241 | Validate GC1sa is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005234 | Validate GC1sd is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005227 | Validate GC1ri is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005210 | Validate GC1ro is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005203 | Validate GC1bc is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005197 | Validate GC1au is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005180 | Validate GC1ab is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005142 | Validate Fibrinogen is intended for the quantitative determination of Calibration Verification/L |
| 00859110005135 | Validate Fibrinogen is intended for the quantitative determination of Calibration Verification/L |
| 00859110005128 | Validate Fibrinogen is intended for the quantitative determination of Calibration Verification/L |
| 00859110005111 | Validate Heparin is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005104 | Validate Heparin is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005098 | Validate Heparin is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005081 | Validate D-Dimer is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005074 | Validate D-Dimer is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005067 | Validate D-Dimer is intended for the quantitative determination of Calibration Verification/Line |
| 00859110005050 | Validate TDM1vt is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005043 | Validate TDM1ri is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005036 | Validate TDM1db is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005029 | Validate TDM1bc is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005012 | Validate TDM1au is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005005 | Validate TDM1ab is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005418 | Validate GC4 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005968 | Validate UC1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005876 | Validate SP1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005838 | Validate Vit D is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005814 | Validate Fertility 1 is intended for the quantitative determination of Calibration Verification/ |
| 00859110005746 | Validate LP is intended for the quantitative determination of Calibration Verification/Linearity |
| 00859110005630 | Validate CM2 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005593 | Validate CM1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005579 | Validate Anemia is intended for the quantitative determination of Calibration Verification/Linea |
| 00859110005548 | Validate Body Fluids is intended for the quantitative determination of Calibration Verification/ |
| 00859110005517 | Validate IBC is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005340 | Validate GC3 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005289 | Validate GC2 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005272 | Validate GC2 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00850011135174 | Validate THY is intended for the quantitative determination of Calibration Verification/Linearit |
| 00850011135105 | Validate UC5 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00850011135044 | Validate UC4 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005999 | Validate UC1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005982 | Validate UC1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005975 | Validate UC1 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005951 | Validate Whole Blood Glucose (WBG) is intended for the quantitative determination of Calibration |
| 00859110005944 | Validate HbA1c is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005937 | Validate HbA1c is intended for the quantitative determination of Calibration Verification/Linear |
| 00859110005920 | Validate SP3 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005913 | Validate SP2 is intended for the quantitative determination of Calibration Verification/Linearit |
| 00859110005906 | Validate SP2 is intended for the quantitative determination of Calibration Verification/Linearit |