ONE TRAY® Sealed Sterilization Container
GUDID 00859522006126
INNOVATIVE STERILIZATION TECHN
Sterilization packaging, reusable| Primary Device ID | 00859522006126 |
| NIH Device Record Key | 4bec7aa4-7380-4c7f-8bcf-b989c70054cb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ONE TRAY® Sealed Sterilization Container |
| Version Model Number | M2108 |
| Company DUNS | 078885794 |
| Company Name | INNOVATIVE STERILIZATION TECHN |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00859522006126 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K052567
FDA Product Code
| KCT | Sterilization Wrap Containers, Trays, Cassettes & Other Accessories |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
[00859522006126]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-11-08 |
| Device Publish Date | 2016-09-24 |
On-Brand Devices [ONE TRAY® Sealed Sterilization Container]
| 00859522006638 | ASSY-2445 |
| 00859522006621 | ASSY-2425 |
| 00859522006614 | ASSY-2145 |
| 00859522006607 | ASSY-2125 |
| 00859522006539 | ASSY-2435 |
| 00859522006522 | ASSY-2415 |
| 00859522006515 | ASSY-2135 |
| 00859522006508 | ASSY-2115 |
| 00859522006409 | CASE-1000-13 |
| 00859522006157 | M2408 |
| 00859522006140 | M2406 |
| 00859522006133 | M2404 |
| 00859522006126 | M2108 |
| 00859522006119 | M2106 |
| 00859522006102 | M2104 |
| 00859522006058 | (L) 23.142" x (H) 8.573" x (W) 11.616" |
| 00859522006041 | (L) 23.142" x (H) 6.573" x (W) 11.616" |
| 00859522006034 | (L) 23.142" x (H) 4.573" x (W) 11.616" |
| 00859522006027 | (L) 20.142" x (H) 8.573" x (W) 11.616" |
| 00859522006003 | (L) 20.142" x (H) 4.573" x (W) 11.616" |
| 00859522006072 | OTTGREEN400 |
| 00859522006065 | OTTORANGE400 |
Trademark Results [ONE TRAY]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
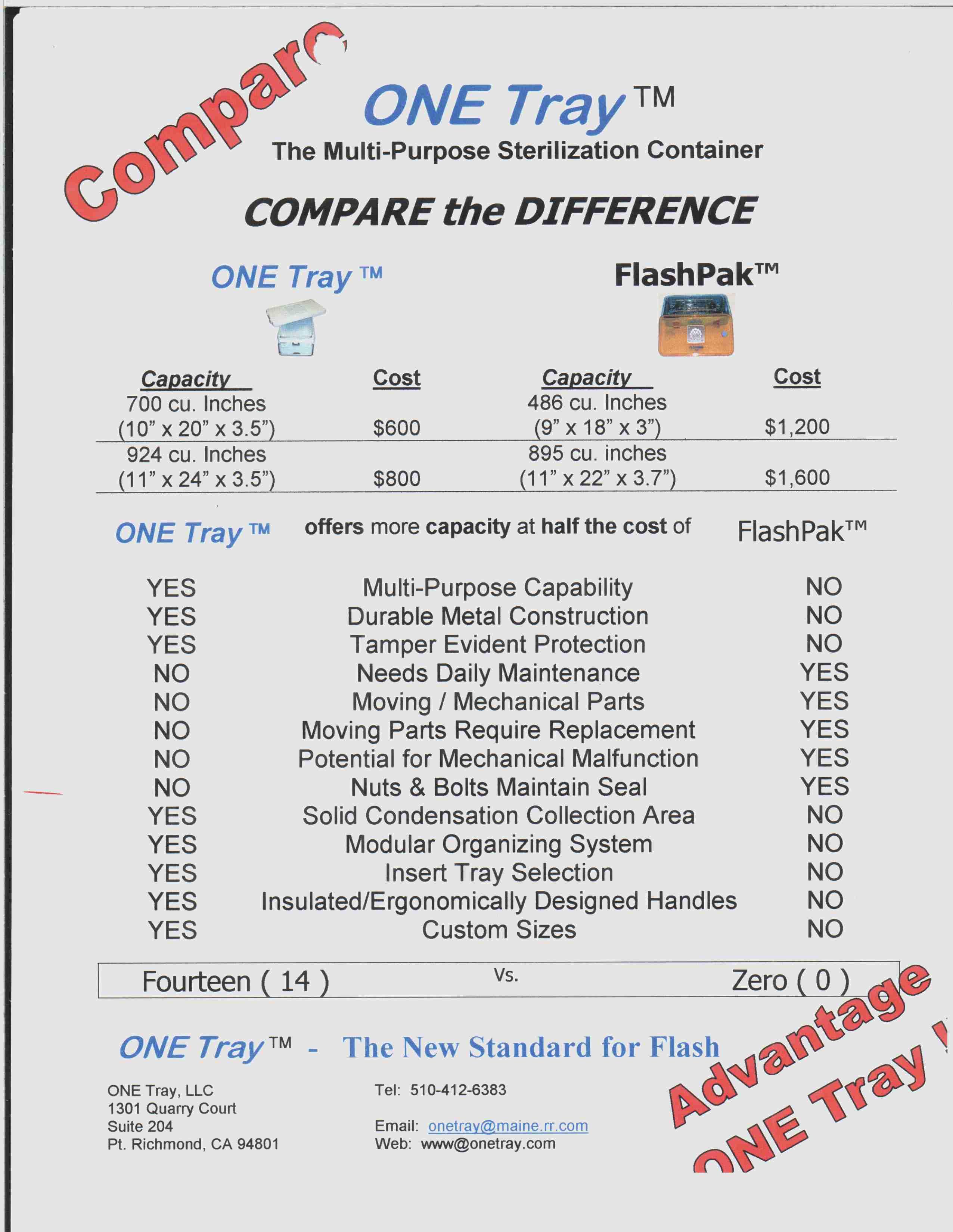 ONE TRAY 78108889 2772642 Live/Registered |
INNOVATIVE STERILIZATION TECHNOLOGIES, LLC 2002-02-14 |
 ONE TRAY 75950207 not registered Dead/Abandoned |
INNOVATIVE STERILIZATION TECHNOLOGIES, LLC 2000-02-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.