V-Tip BL-VTR-100
GUDID 00860004701811
BIO-LIFE INNOVATIONS, LLC
Airway suction catheter| Primary Device ID | 00860004701811 |
| NIH Device Record Key | 45b318df-1b52-4652-8901-cbd055c7acb3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | V-Tip |
| Version Model Number | VTR-100 |
| Catalog Number | BL-VTR-100 |
| Company DUNS | 117588031 |
| Company Name | BIO-LIFE INNOVATIONS, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Customer Support Contacts
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com | |
| Phone | (330)453-9710 |
| kthomas@bio-lifeinnovations.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00860004701804 [Primary] |
| GS1 | 00860004701811 [Package] Contains: 00860004701804 Package: Carton [48 Units] In Commercial Distribution |
| GS1 | 00860004701828 [Package] Package: Case [4 Units] In Commercial Distribution |
FDA Product Code
| JOL | Catheter And Tip, Suction |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2020-08-26 |
| Device Publish Date | 2020-08-18 |
Trademark Results [V-Tip]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
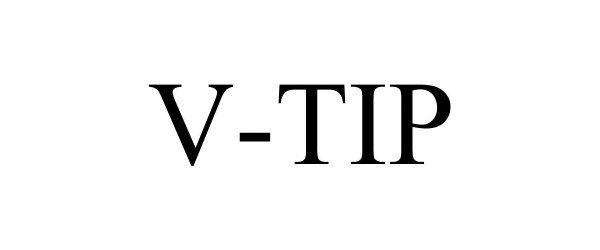 V-TIP 90071163 not registered Live/Pending |
Bio-Life Innovations, LLC 2020-07-24 |
 V-TIP 86849980 not registered Dead/Abandoned |
Hirsch, Julian 2015-12-15 |
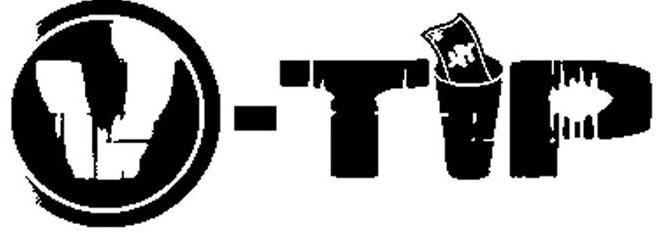 V-TIP 77268827 3551203 Dead/Cancelled |
VTIP ENTERTAINMENT, LLC 2007-08-30 |
 V-TIP 76545320 2937083 Dead/Cancelled |
Swemed Lab International AB 2003-09-16 |
 V-TIP 72164672 0766197 Live/Registered |
Vernay Laboratories, Inc. 1963-03-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.