qER-Quant
GUDID 00860005118137
The qER-Quant device is intended for automatic labeling, visualization and quantification of segmentable brain structures from a set of Non-Contrast head CT (NCCT) images. The software is intended to automate the current manual process of identifying, labeling and quantifying the volume of segmentable brain structures identified on NCCT images.
QURE.AI TECHNOLOGIES PRIVATE LIMITED
Radiology DICOM image processing application software| Primary Device ID | 00860005118137 |
| NIH Device Record Key | 52aeba6c-5c3e-4371-8882-d09b5685f23e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | qER-Quant |
| Version Model Number | 1.0.0 |
| Company DUNS | 860393884 |
| Company Name | QURE.AI TECHNOLOGIES PRIVATE LIMITED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai | |
| Phone | +1(262)347-9734 |
| support@qure.ai |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00860005118137 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K211222
FDA Product Code
| QIH | Automated Radiological Image Processing Software |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-06-08 |
| Device Publish Date | 2022-05-31 |
Devices Manufactured by QURE.AI TECHNOLOGIES PRIVATE LIMITED
| 00850057636024 - qXR-LN | 2024-07-02 The qXR-LN (qXR_Lung_nodule) is computer-aided detection software to identify and mark regions in relation to suspected pulmonar |
| 00850057636000 - qXR-CTR | 2023-10-18 qXR-CTR is a deep-learning based software for use by hospitals and clinics for automated assessment of the CTR on chest X-ray (C |
| 00850057636017 - qXR-PTX-PE | 2023-10-18 qXR-PTX-PE is a radiological computer-assisted triage and notification software that analyzes adult chest X-ray images for the p |
| 00860005118137 - qER-Quant | 2022-06-08The qER-Quant device is intended for automatic labeling, visualization and quantification of segmentable brain structures from a set of Non-Contrast head CT (NCCT) images. The software is intended to automate the current manual process of identifying, labeling and quantifying the volume of segmentable brain structures identified on NCCT images. |
| 00860005118137 - qER-Quant | 2022-06-08 The qER-Quant device is intended for automatic labeling, visualization and quantification of segmentable brain structures from a |
| 00860005118144 - qXR-BT | 2022-06-08 The qXR-BT device is intended to generate a secondary digital chest X-ray image that facilitates confirmation of the position of |
| 00860005118106 - qER | 2020-12-14 Qure.ai Head CT scan interpretation software, qER, is a deep-learning-based software device that analyses head CT scans for sign |
Trademark Results [qER-Quant]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
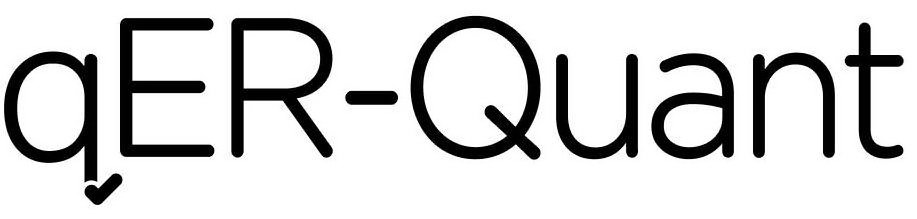 QER-QUANT 97287368 not registered Live/Pending |
Qure.ai Technologies Private Limited 2022-02-28 |
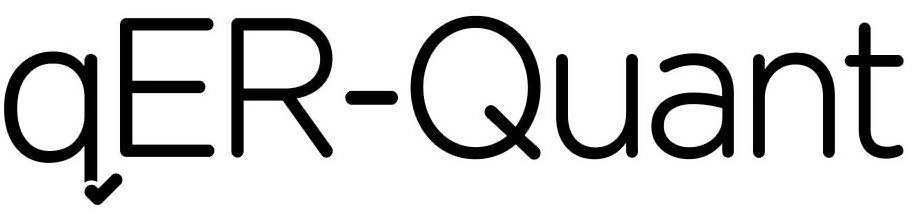 QER-QUANT 97287357 not registered Live/Pending |
Qure.ai Technologies Private Limited 2022-02-28 |
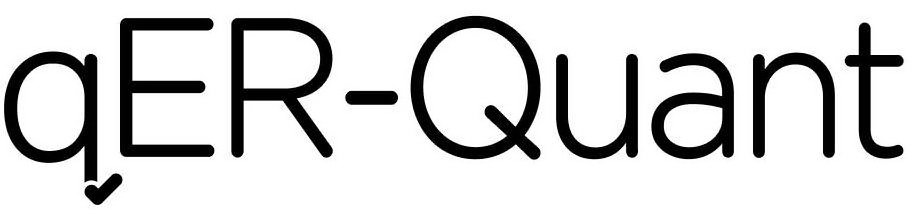 QER-QUANT 97287349 not registered Live/Pending |
Qure.ai Technologies Private Limited 2022-02-28 |
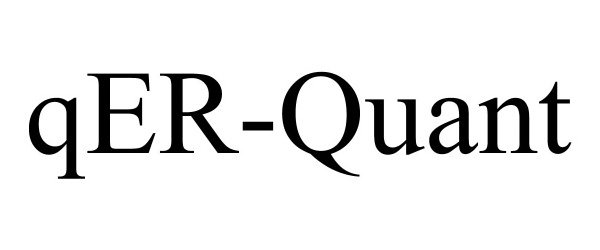 QER-QUANT 97217964 not registered Live/Pending |
Qure.ai Technologies Private Limited 2022-01-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.