GERSON 083230+
GUDID 00860006025762
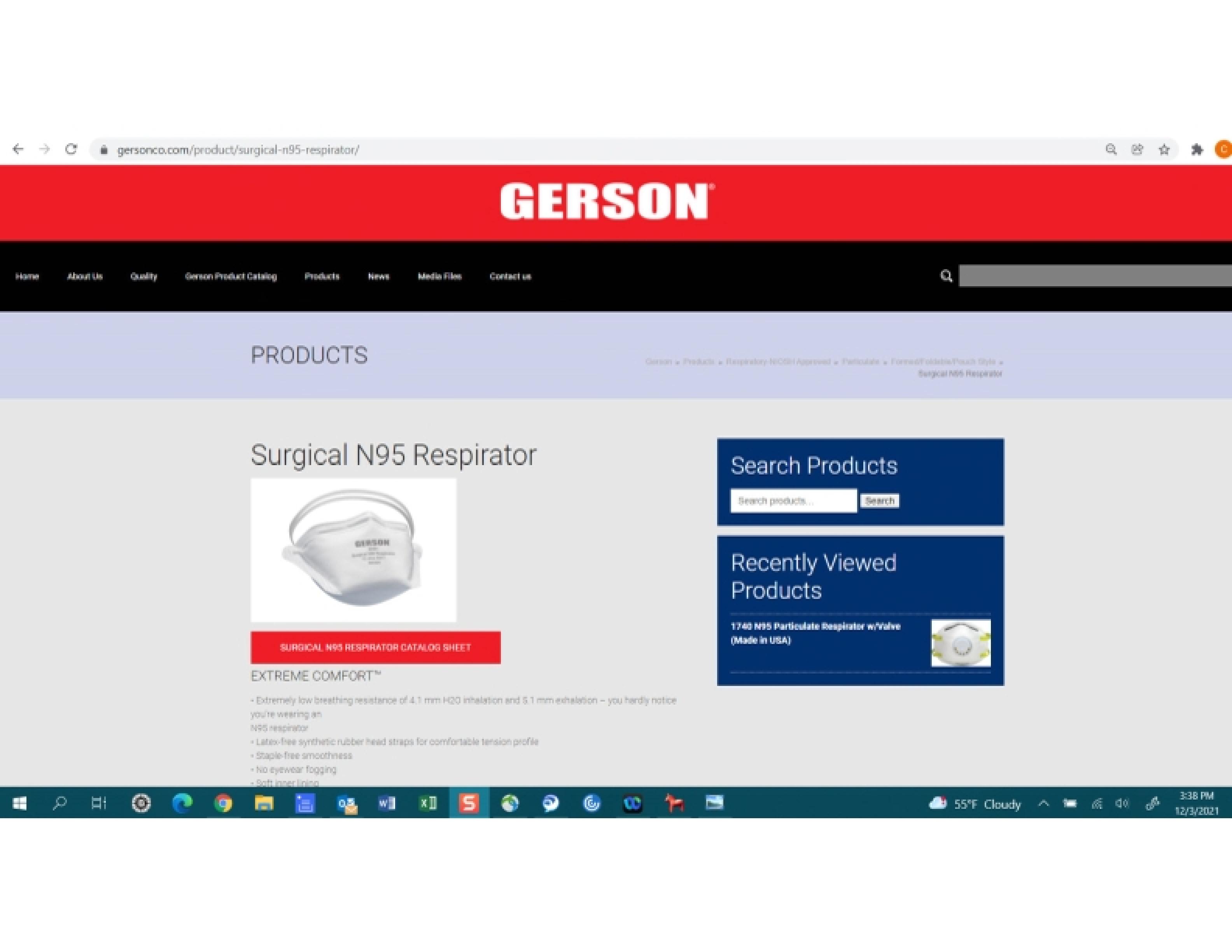
Surgical N95 Respirator
LOUIS M. GERSON CO.
Surgical/medical respirator, non-antimicrobial, single-use| Primary Device ID | 00860006025762 |
| NIH Device Record Key | ec060629-b120-406a-98fc-361d9604ec1e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | GERSON |
| Version Model Number | 3230+ |
| Catalog Number | 083230+ |
| Company DUNS | 001000363 |
| Company Name | LOUIS M. GERSON CO. |
| Device Count | 50 |
| DM Exempt | true |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00860006025762 [Primary] |
| GS1 | 00860006025779 [Package] Package: Case [4 Units] In Commercial Distribution |
| GS1 | 00860006025793 [Unit of Use] |
FDA Product Code
| MSH | Respirator, Surgical |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-11-08 |
| Device Publish Date | 2022-10-31 |
On-Brand Devices [GERSON]
| 00860006025762 | Surgical N95 Respirator |
| 00044585917308 | 1730 Type N95 Particulate Respirator and Surgical Respirator |
| 00044585833301 | Surgical N95 Respirator |
Trademark Results [GERSON]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GERSON 97712145 not registered Live/Pending |
Louis M. Gerson Co., Inc. 2022-12-10 |
 GERSON 88384910 not registered Live/Pending |
Natural Weight Loss and Pharmacy, Inc 2019-04-14 |
 GERSON 75127226 2526365 Live/Registered |
GERSON INSTITUTE 1996-06-28 |
 GERSON 75014817 2521443 Live/Registered |
Gerson Institute 1995-11-03 |
 GERSON 75013108 not registered Dead/Abandoned |
Rogers, Dan E. 1995-10-31 |
 GERSON 74735124 2082964 Live/Registered |
LOUIS M. GERSON, INC. 1995-09-28 |
 GERSON 73260845 1213017 Live/Registered |
Louis M. Gerson Co., Inc. 1980-05-05 |
 GERSON 72462068 1005547 Live/Registered |
LOUIS M. GERSON CO., INC. 1973-07-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.