UpLyft
GUDID 00860009140509
Patient Transfer System
PATHWAY, LLC
Mobile patient lifting system, electrically-powered| Primary Device ID | 00860009140509 |
| NIH Device Record Key | 301ffdb8-70b2-4499-943f-064d3cd4cb2b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | UpLyft |
| Version Model Number | ST-120 |
| Company DUNS | 080312381 |
| Company Name | PATHWAY, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00860009140509 [Primary] |
FDA Product Code
| FSA | Lift, Patient, Non-Ac-Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-08-16 |
| Device Publish Date | 2023-08-08 |
Devices Manufactured by PATHWAY, LLC
| 00850041415130 - ClearTract | 2024-05-29 2-Way 100% Silicone, 14Fr X 10ml, ClearTract SPT Catheter |
| 00850041415147 - ClearTract | 2024-05-29 2-Way 100% Silicone, 16Fr X 5ml, ClearTract SPT Catheter |
| 00850041415154 - ClearTract | 2024-05-29 2-Way 100% Silicone, 16Fr X 10ml, ClearTract SPT Catheter |
| 00850041415161 - ClearTract | 2024-05-29 2-Way 100% Silicone, 18Fr X 10ml, ClearTract SPT Catheter |
| 00860009140509 - UpLyft | 2023-08-16Patient Transfer System |
| 00860009140509 - UpLyft | 2023-08-16 Patient Transfer System |
| 00851682007562 - Cooler Heads | 2022-11-15 AMMA Portable Scalp Cooling System |
| 20851682007009 - Kurin Blood Collection Set, 21 Ga, BioMerieux | 2019-10-23 |
| 20851682007016 - Kurin Blood Collection Set, 23 Ga, BD BioMerieux | 2019-10-23 |
Trademark Results [UpLyft]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 UPLYFT 97674412 not registered Live/Pending |
DME Innovations, Inc. 2022-11-11 |
 UPLYFT 97407841 not registered Live/Pending |
Component Sourcing International, LLC 2022-05-12 |
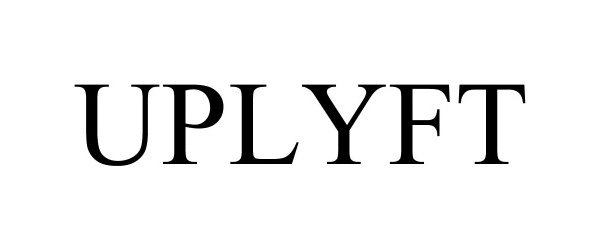 UPLYFT 97405716 not registered Live/Pending |
Component Sourcing International, LLC 2022-05-11 |
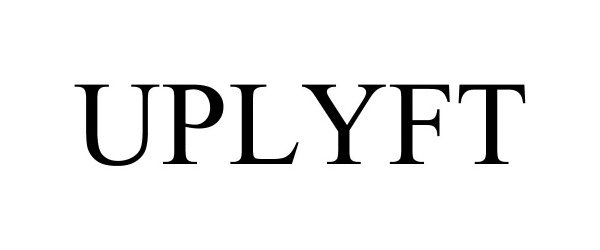 UPLYFT 97326633 not registered Live/Pending |
Masterack Manufacturing and Installation LLC 2022-03-23 |
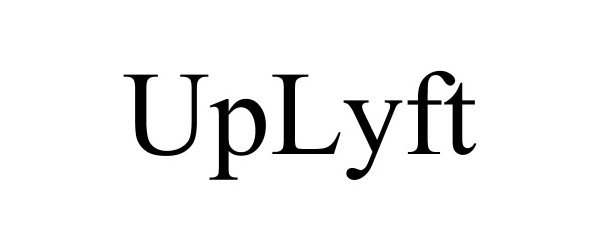 UPLYFT 88830660 not registered Live/Pending |
DME Innovations, Inc. 2020-03-11 |
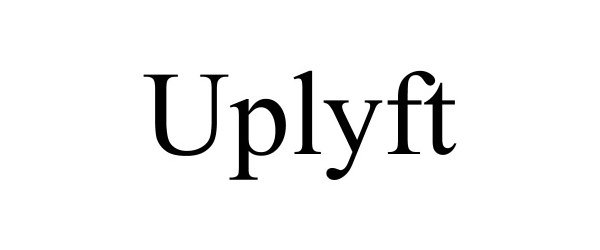 UPLYFT 88818023 not registered Live/Pending |
Lear, Erin 2020-03-03 |
 UPLYFT 88301539 not registered Dead/Abandoned |
British American Tobacco (Brands) Limited 2019-02-14 |
 UPLYFT 88212928 not registered Dead/Abandoned |
Uplyft Enterprises, LLC 2018-11-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.