ImageMax
GUDID 00862879000303
Automatic X-Ray Film Processor
TATE TECHNOLOGY, INC.
Dental automated x-ray film processor| Primary Device ID | 00862879000303 |
| NIH Device Record Key | 604c0d66-4e78-45a5-b1f3-b108315b3186 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ImageMax |
| Version Model Number | 950 |
| Company DUNS | 789331063 |
| Company Name | TATE TECHNOLOGY, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00862879000303 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K992896
FDA Product Code
| EGY | Processor, Radiographic-Film, Automatic, Dental |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-11-01 |
Trademark Results [ImageMax]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
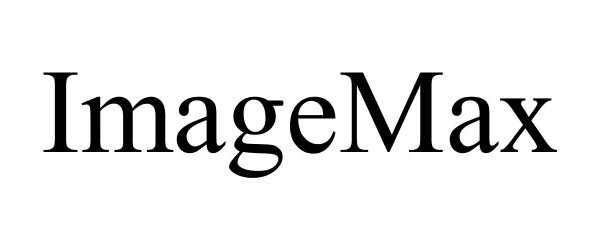 IMAGEMAX 86255047 4644855 Live/Registered |
D&E Technology 2014-04-17 |
 IMAGEMAX 78718440 not registered Dead/Abandoned |
Speedpro USA, Inc. 2005-09-22 |
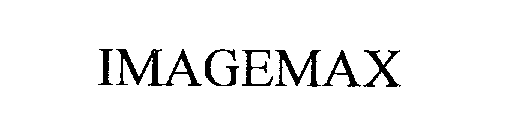 IMAGEMAX 76278615 2743731 Dead/Cancelled |
Thermo Nicolet Corporation 2001-06-29 |
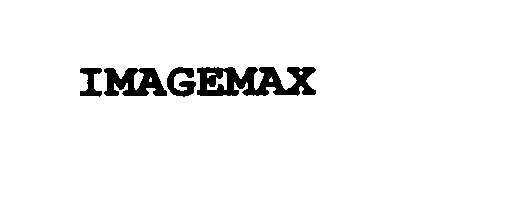 IMAGEMAX 75852258 not registered Dead/Abandoned |
PHILIPS ELECTRONICS NORTH AMERICA CORPORATION 1999-11-18 |
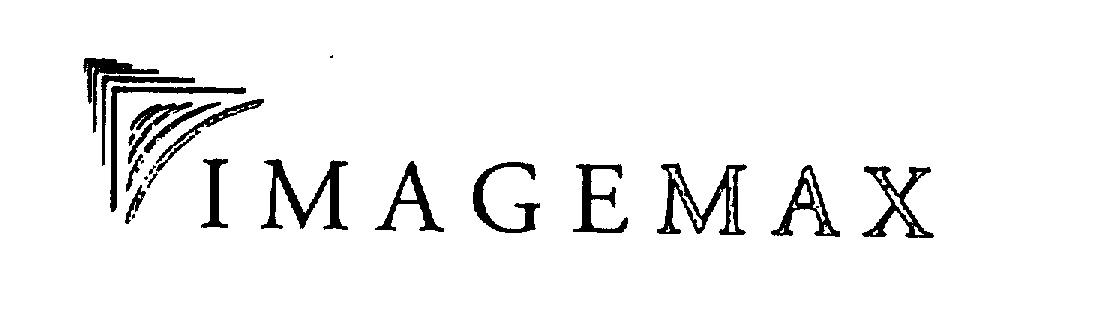 IMAGEMAX 75469442 2317912 Dead/Cancelled |
DataBank IMX LLC 1998-04-17 |
 IMAGEMAX 75359347 2414922 Live/Registered |
DATABANK IMX LLC 1997-09-18 |
 IMAGEMAX 75104198 2098975 Live/Registered |
DATABANK IMX LLC 1996-05-13 |
 IMAGEMAX 74398203 not registered Dead/Abandoned |
MAKTAL, INC. 1993-06-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.