BI-POLAR 31-479571
GUDID 00880304319943
Biomet Orthopedics, LLC
Femoral head prosthesis trial| Primary Device ID | 00880304319943 |
| NIH Device Record Key | 61f34111-1b75-4ccb-9a88-e40d923d092a |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | BI-POLAR |
| Version Model Number | 31-479571 |
| Catalog Number | 31-479571 |
| Company DUNS | 129278169 |
| Company Name | Biomet Orthopedics, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00880304319943 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K051569
FDA Product Code
| KWY | Prosthesis, hip, hemi-, femoral, metal/polymer, cemented or uncemented |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
[00880304319943]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-02-07 |
| Device Publish Date | 2016-09-22 |
On-Brand Devices [BI-POLAR]
| 00880304319943 | 31-479571 |
| 00880304319912 | 31-479564 |
| 00880304319899 | 31-479562 |
| 00880304319875 | 31-479560 |
| 00880304319868 | 31-479559 |
| 00880304319844 | 31-479557 |
| 00880304319837 | 31-479556 |
| 00880304319813 | 31-479554 |
| 00880304319790 | 31-479552 |
| 00880304319479 | 31-479365 |
| 00880304319462 | 31-479285 |
| 00887868481468 | 31-479365 |
| 00887868479144 | 31-401137 |
| 00887868479137 | 31-401136 |
| 00887868479120 | 31-401135 |
| 00887868479113 | 31-401134 |
| 00887868479106 | 31-401133 |
| 00887868479090 | 31-401132 |
| 00887868479083 | 31-401131 |
| 00880304567740 | 110005124 |
| 00880304567733 | 110005123 |
| 00880304567665 | 110005109 |
Trademark Results [BI-POLAR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BI-POLAR 87175627 not registered Dead/Abandoned |
Jackson, Victoria 2016-09-19 |
 BI-POLAR 87175627 not registered Dead/Abandoned |
Guthy, Jackson 2016-09-19 |
 BI-POLAR 85940528 4516329 Live/Registered |
Air Oasis LTD 2013-05-23 |
 BI-POLAR 77168187 not registered Dead/Abandoned |
Wasson, James 2007-04-27 |
 BI-POLAR 77168187 not registered Dead/Abandoned |
Creagan, Dave 2007-04-27 |
 BI-POLAR 76502363 2810359 Dead/Cancelled |
Designer Skin, LLC 2003-03-31 |
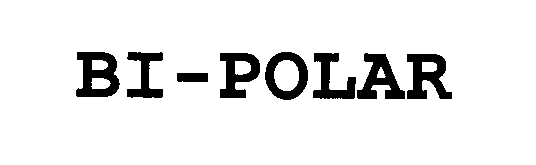 BI-POLAR 76391297 not registered Dead/Abandoned |
Polar Design, Inc. 2002-04-03 |
 BI-POLAR 75593902 2830256 Dead/Cancelled |
GFTA ANALYTICS LTD. 1998-11-23 |
 BI-POLAR 75464266 2226234 Live/Registered |
BPG, LLC 1998-04-08 |
 BI-POLAR 74688442 not registered Dead/Abandoned |
Emhart Inc. 1995-06-15 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.