Input® SSV 808700
GUDID 00884450505946
Merit Medical Systems, Inc.
Vascular catheter introduction set, nonimplantable| Primary Device ID | 00884450505946 |
| NIH Device Record Key | b82a9550-bff8-4f7f-914e-e109d8c316c0 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Input® SSV |
| Version Model Number | 10884450505943 |
| Catalog Number | 808700 |
| Company DUNS | 184763290 |
| Company Name | Merit Medical Systems, Inc. |
| Device Count | 5 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884450505946 [Unit of Use] |
| GS1 | 10884450505943 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K934901
FDA Product Code
| DYB | INTRODUCER, CATHETER |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-08-09 |
| Device Publish Date | 2021-07-30 |
On-Brand Devices [Input® SSV]
| 00884450506059 | 10884450506056 |
| 00884450506035 | 10884450506032 |
| 00884450506028 | 10884450506025 |
| 00884450506011 | 10884450506018 |
| 00884450506004 | 10884450506001 |
| 00884450505991 | 10884450505998 |
| 00884450505984 | 10884450505981 |
| 00884450505946 | 10884450505943 |
| 00884450506042 | 10884450506049 |
Trademark Results [Input]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 INPUT 97447940 not registered Live/Pending |
El-Ghawaby, Mohab 2022-06-08 |
 INPUT 88524916 not registered Live/Pending |
BDG Media, Inc. 2019-07-19 |
 INPUT 87978649 5603411 Live/Registered |
Input Tech, Inc. 2017-02-24 |
 INPUT 87349048 not registered Live/Pending |
Input Tech, Inc. 2017-02-24 |
 INPUT 87017908 not registered Dead/Abandoned |
Peter Cunningham 2016-04-28 |
 INPUT 86217526 4617376 Live/Registered |
THE FONT BUREAU INC. 2014-03-11 |
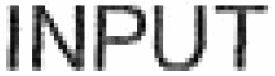 INPUT 79289760 not registered Live/Pending |
SEI-HEI MOON, S.L. 2020-06-19 |
 INPUT 78203132 2788957 Dead/Cancelled |
INPUT, INC. 2003-01-14 |
 INPUT 77770848 3990598 Dead/Cancelled |
Krueger International, Inc. 2009-06-30 |
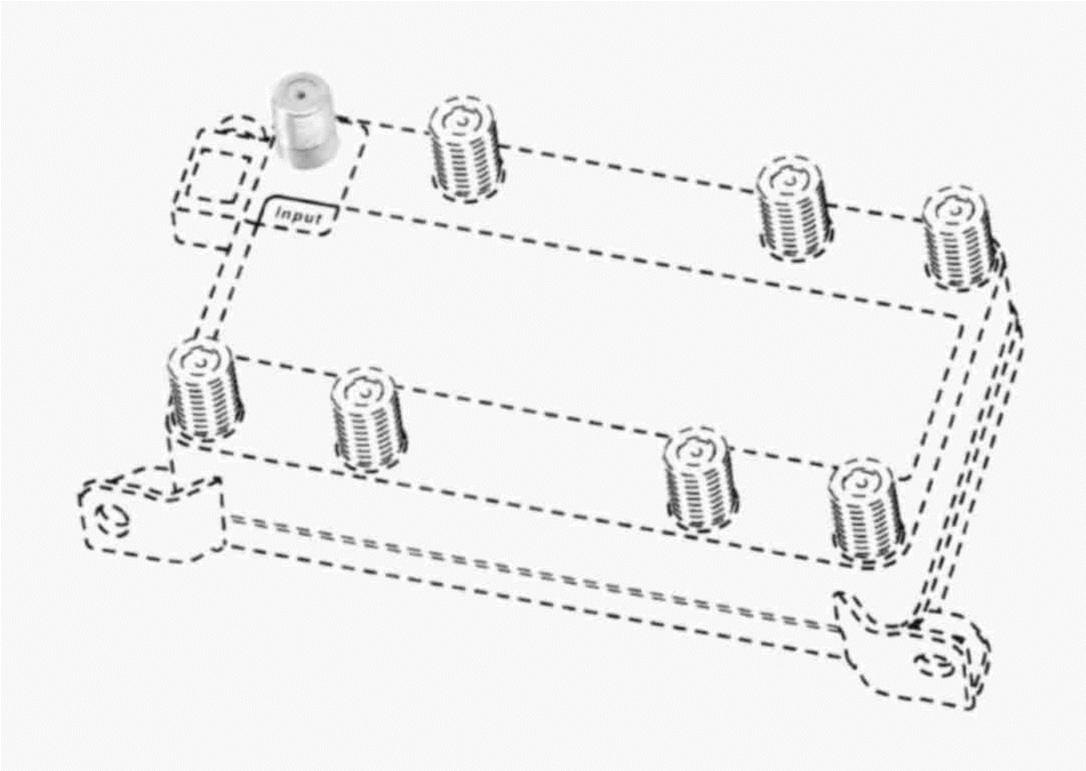 INPUT 76714207 4479134 Live/Registered |
TIMES FIBER COMMUNICATIONS, INC. 2013-05-23 |
 INPUT 76188467 2946849 Live/Registered |
Gallup, Inc. 2001-01-02 |
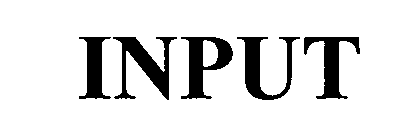 INPUT 75605621 2468218 Dead/Cancelled |
Punch Products USA, Inc. 1998-12-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.