Surfacer® 600200
GUDID 00884450762158
Merit Medical Systems, Inc.
Central venous catheter inside-out introduction set| Primary Device ID | 00884450762158 |
| NIH Device Record Key | f18b92be-6f78-4087-b6ab-a93d4fdb8a17 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Surfacer® |
| Version Model Number | C |
| Catalog Number | 600200 |
| Company DUNS | 184763290 |
| Company Name | Merit Medical Systems, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884450762158 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- deNovo: DEN190038
FDA Product Code
| QJH | Reverse central venous recanalization system |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-03-11 |
| Device Publish Date | 2024-03-02 |
Devices Manufactured by Merit Medical Systems, Inc.
| 10884450027384 - Merit Medical® | 2026-02-23 |
| 20884450029019 - Merit Medical® | 2026-02-23 |
| 10884450031862 - Merit Medical® | 2026-02-23 |
| 20884450101944 - Merit Medical® | 2026-02-23 |
| 20884450111851 - Merit Medical® | 2026-02-23 |
| 10884450112769 - Merit Medical® | 2026-02-23 |
| 10884450121396 - Merit Medical® | 2026-02-23 |
| 20884450161580 - Merit Medical® | 2026-02-23 |
Trademark Results [Surfacer]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SURFACER 85648683 4649588 Live/Registered |
Bluegrass Vascular Technologies Inc. 2012-06-11 |
 SURFACER 79348673 not registered Live/Pending |
ASF METROLOGY S.R.L. 2022-07-29 |
 SURFACER 75115452 not registered Dead/Abandoned |
SANGI CO., LTD. 1996-06-07 |
 SURFACER 74375194 1813546 Dead/Cancelled |
UGS PLM SOLUTIONS INC. 1993-04-05 |
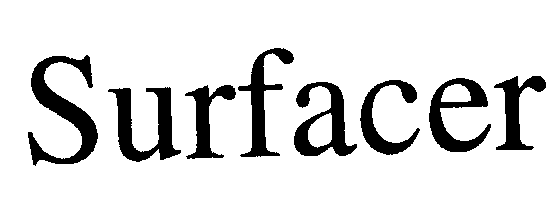 SURFACER 74264362 not registered Dead/Abandoned |
IMAGEWARE 1992-04-09 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.