MicroDose SI 714248
GUDID 00884838071001
Philips MicroDose SI (Model: L50) is intended to produce radiographic images of the human breast for the purpose of diagnostic and screening mammography. The system is intended to be used in the same clinical applications as traditional film-based mammography systems.
Philips Digital Mammography Sweden AB
Stationary mammographic x-ray system, digital| Primary Device ID | 00884838071001 |
| NIH Device Record Key | 988c3386-8732-4f7e-97ba-7ebdb8ce7645 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MicroDose SI |
| Version Model Number | L50 |
| Catalog Number | 714248 |
| Company DUNS | 632899233 |
| Company Name | Philips Digital Mammography Sweden AB |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00884838071001 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K130704
FDA Product Code
| MUE | Full field digital,system,x-ray,mammographic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-11-12 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [MicroDose SI]
| 00884838071001 | Philips MicroDose SI (Model: L50) is intended to produce radiographic images of the human breast |
| 00884838070943 | Philips MicroDose Mammography SI L50 is intended to produce radiographic images of the human bre |
Trademark Results [MicroDose SI]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
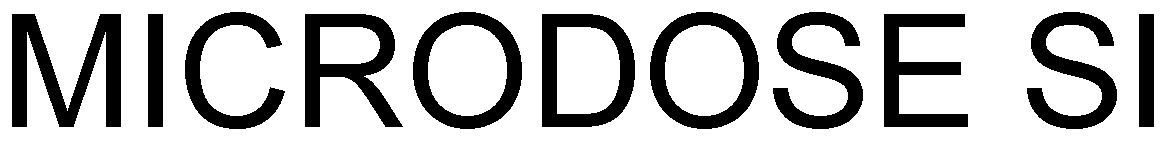 MICRODOSE SI 79131247 4661406 Live/Registered |
Koninklijke Philips N.V. 2013-04-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.