RESUME® TL
GUDID 00885074471969
LEAD 3986A45 RESUME T IN-LINE 45CM ID
MEDTRONIC, INC.
Neural-tissue electrical stimulation lead| Primary Device ID | 00885074471969 |
| NIH Device Record Key | cb25d1f9-d8cf-4743-9f3c-fd1fec4cf833 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | RESUME® TL |
| Version Model Number | 3986A45 |
| Company DUNS | 796986144 |
| Company Name | MEDTRONIC, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
| Length | 45 Centimeter |
Operating and Storage Conditions
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
| Storage Environment Temperature | Between -30 Degrees Fahrenheit and 135 Degrees Fahrenheit |
| Storage Environment Temperature | Between -34 Degrees Celsius and 57 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00885074471969 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P840001
FDA Product Code
| LGW | STIMULATOR, SPINAL-CORD, TOTALLY IMPLANTED FOR PAIN RELIEF |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2014-09-23 |
On-Brand Devices [RESUME® TL]
| 00885074478968 | LEAD 3986A60 RESUME TL IN-LINE 60CM ID |
| 00885074471976 | LEAD 3986A70 RESUME T IN-LINE 70CM ID |
| 00885074471969 | LEAD 3986A45 RESUME T IN-LINE 45CM ID |
Trademark Results [RESUME]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
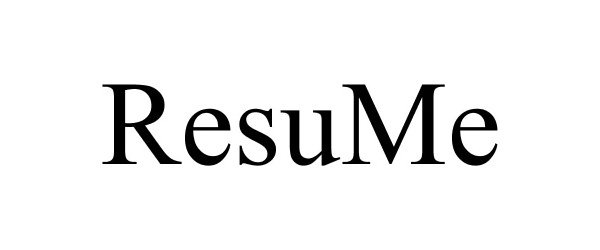 RESUME 87525136 not registered Live/Pending |
Gregory, Rex 2017-07-12 |
 RESUME 86555188 not registered Dead/Abandoned |
Resume, Inc. 2015-03-05 |
 RESUME 85922447 not registered Dead/Abandoned |
Goldstein, Ira 2013-05-03 |
 RESUME 85922447 not registered Dead/Abandoned |
Goldstein, David 2013-05-03 |
 RESUME 85171756 4099996 Dead/Cancelled |
Celgene Corporation 2010-11-08 |
 RESUME 85047318 3940196 Live/Registered |
Celgene Corporation 2010-05-25 |
 RESUME 79199138 5220557 Live/Registered |
QUANTEL MEDICAL 2016-09-08 |
 RESUME 77178504 3365422 Dead/Cancelled |
Medtronic, Inc. 2007-05-11 |
 RESUME 75107020 not registered Dead/Abandoned |
Private Portfolio, Inc. 1996-05-20 |
 RESUME 74256797 not registered Dead/Abandoned |
MERCK & CO., Inc. 1992-03-18 |
 RESUME 73486604 1367539 Dead/Cancelled |
TALBOTS, INC., THE 1984-06-22 |
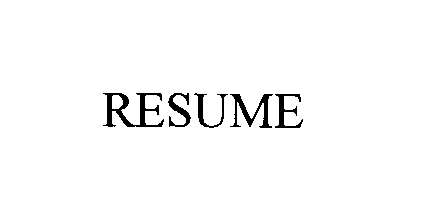 RESUME 73388193 1266240 Dead/Cancelled |
MEDTRONIC, INC. 1982-09-23 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.