TRAUMA E0017320-1
GUDID 00885556804834
205MM FORCEPS W/BALL TIP
Smith & Nephew, Inc.
Surgical implant handling forceps| Primary Device ID | 00885556804834 |
| NIH Device Record Key | 84d883e7-173a-48ca-8917-83531fe38258 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TRAUMA |
| Version Model Number | E0017320-1 |
| Catalog Number | E0017320-1 |
| Company DUNS | 109903521 |
| Company Name | Smith & Nephew, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM | |
| Phone | +1(800)238-7538 |
| GUDID@SMITH-NEPHEW.COM |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00885556804834 [Primary] |
FDA Product Code
| HTD | FORCEPS |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
[00885556804834]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-03-29 |
| Device Publish Date | 2021-03-19 |
On-Brand Devices [TRAUMA]
| 00885556704929 | K-WIRE GUIDE WITH LOOP |
| 00885556692738 | MODIFIED CONQUEST FN PROXIMAL SCREWDRIVER |
| 00885556684801 | LONG T8 DRIVER WITH AO QUICK CONNECT |
| 00885556684795 | LONG T7 DRIVER WITH AO QUICK CONNECT |
| 00885556684788 | LONG T6 DRIVER WITH AO QUICK CONNECT |
| 00885556683606 | MODIFIED 22MM CONE REAMER |
| 00885556683590 | MODIFIED 20MM CONE REAMER |
| 00885556683583 | MODIFIED 18MM CONE REAMER |
| 00885556683576 | MODIFIED 16MM CONE REAMER |
| 00885556683569 | MODIFIED 14MM CONE REAMER |
| 00885556683361 | MODIFIED 22MM CONE REAMER |
| 00885556683354 | MODIFIED 20MM CONE REAMER |
| 00885556683347 | MODIFIED 18MM CONE REAMER |
| 00885556683330 | MODIFIED 16MM CONE REAMER |
| 00885556683323 | MODIFIED 14MM CONE REAMER |
| 00885556804322 | CANNULATED DEPTH GAUGE |
| 00885556804834 | 205MM FORCEPS W/BALL TIP |
| 00885556892480 | DIRECT MEASURING GUIDE PIN SLEEVE |
Trademark Results [TRAUMA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TRAUMA 97929696 not registered Live/Pending |
RTSD Wards LLC 2023-05-10 |
 TRAUMA 88221927 not registered Live/Pending |
Hogue Tool & Machine, Inc. 2018-12-08 |
 TRAUMA 86664289 4881619 Live/Registered |
Ward, Dennis 2015-06-16 |
 TRAUMA 85694941 not registered Dead/Abandoned |
Don Hillier 2012-08-03 |
 TRAUMA 85694941 not registered Dead/Abandoned |
Kris Gustofson 2012-08-03 |
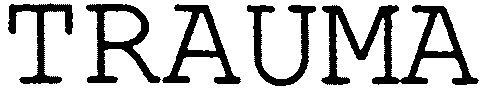 TRAUMA 79036080 3312972 Dead/Cancelled |
ANÃBAL JOSÃ SIMÃES COUTINHO 2006-12-28 |
 TRAUMA 77716006 3832748 Live/Registered |
The New York Times Company 2009-04-17 |
 TRAUMA 77713892 not registered Dead/Abandoned |
Zombie, Inc. 2009-04-14 |
 TRAUMA 75297533 2197524 Live/Registered |
NEW YORK TIMES COMPANY, THE 1997-05-23 |
 TRAUMA 74698323 not registered Dead/Abandoned |
DOVER, JESSE 1995-06-15 |
 TRAUMA 74216936 not registered Dead/Abandoned |
Ravida, Jodi A. 1991-10-30 |
 TRAUMA 73652279 1482766 Dead/Cancelled |
YORK HOSPITAL 1987-03-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.