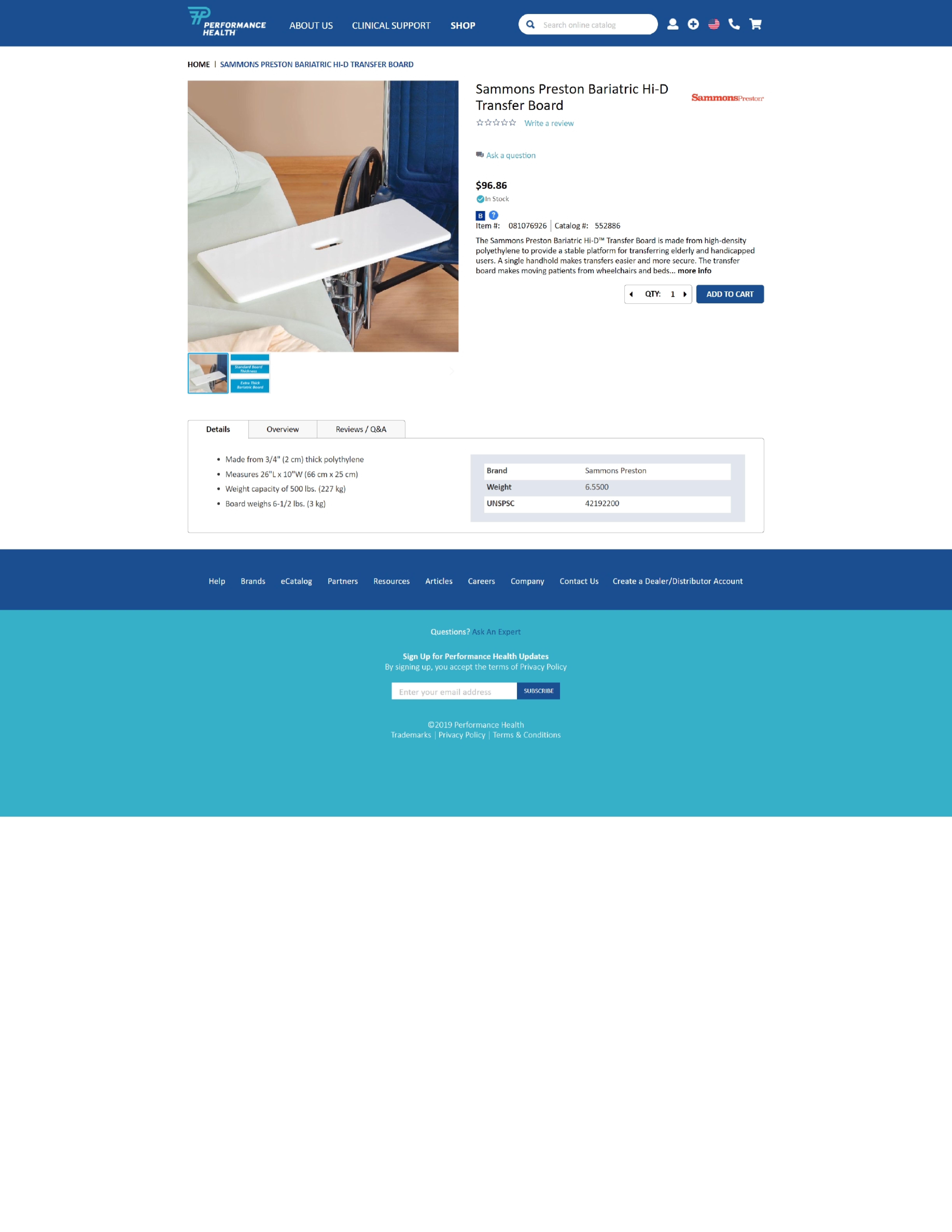
Sammons Preston
GUDID 00885914103524
Sammons Preston Moist Heat Pad, Small 7" x 15"
Performance Health Supply, LLC
Electric pad localized-body heating system| Primary Device ID | 00885914103524 |
| NIH Device Record Key | cd0c3cac-52b6-4133-9d87-353110df77e5 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Sammons Preston |
| Version Model Number | 7011158 |
| Company DUNS | 966806150 |
| Company Name | Performance Health Supply, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | true |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00885914103524 [Primary] |
FDA Product Code
| IRT | Pad, Heating, Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-10-14 |
| Device Publish Date | 2024-10-04 |
On-Brand Devices [Sammons Preston]
| 00885914088159 | Sammons Preston Moist Heat Pad |
| 00885914088142 | Sammons Preston Economy Aneroid Sphygmomanometer |
| 00885914103531 | Sammons Preston Moist Heat Pad, Large 14" x 27" |
| 00885914103524 | Sammons Preston Moist Heat Pad, Small 7" x 15" |
| 00885914108079 | Sammons Preston Refillable Ice Bag w/Clamp Closure, 7.25" x 16", EA |
| 00885914108062 | Sammons Preston Refillable Ice Bag w/Clamp Closure, 5.2" x 13.7", EA |
| 00885914108055 | Sammons Preston Infant Transport Mattress, 9.65" x 15.75", EA |
| 00885914108048 | Sammons Preston Instant Hot Pack, Infant Heel, 4"x 4.4", EA |
| 00885914108031 | Sammons Preston Instant Hot Pack, 4" x 9.25", EA |
| 00885914108024 | Sammons Preston Instant Hot Pack, 5.75" x 8.5", EA |
| 00885914108017 | Sammons Preston Instant Hot Pack, 5.75" x 5.75", EA |
| 00885914108000 | Sammons Preston Instant Cold Pack, Direct to Skin, 6.25"x 9.5", EA |
| 00885914107997 | Sammons Preston Instant Cold Pack, Direct to Skin, 5"x 7.5", EA |
| 00885914107980 | Sammons Preston Instant Cold Pack, Perineal, 5.5" x 14.75", EA |
| 00885914107973 | Sammons Preston Instant Cold Pack, 6.5" x 9", EA |
| 00885914107966 | Sammons Preston Instant Cold Pack, 5.5" x 6.25", EA |
| 00885914107898 | Sammons Preston Hot/Cold Combo pack, 10.625" x 6", EA |
Trademark Results [Sammons Preston]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SAMMONS PRESTON 98248640 not registered Live/Pending |
Performance Health Holdings, Inc. 2023-10-31 |
 SAMMONS PRESTON 75308107 2261983 Live/Registered |
PERFORMANCE HEALTH HOLDINGS, INC. 1997-06-13 |
 SAMMONS PRESTON 75301809 2268126 Live/Registered |
PERFORMANCE HEALTH HOLDINGS, INC. 1997-06-02 |
 SAMMONS PRESTON 74728375 not registered Dead/Abandoned |
BISSELL Healthcare Corporation 1995-09-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.