Monolith
GUDID 00887517706218
Monolith Corpectomy Instrument Tray
Nuvasive, Inc.
Sterilization/disinfection container| Primary Device ID | 00887517706218 |
| NIH Device Record Key | b69c0a24-0797-45a6-ad25-5536006f3306 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Monolith |
| Version Model Number | MONOLITHINS |
| Company DUNS | 053950783 |
| Company Name | Nuvasive, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00887517706218 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K143579
FDA Product Code
| KCT | Sterilization wrap containers, trays, cassettes & other accessories |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
[00887517706218]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-09-10 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [Monolith]
| 00887517677730 | Monolith Inserter |
| 00887517677433 | Monolith Calipers |
| 00887517706218 | Monolith Corpectomy Instrument Tray |
| 00887517706201 | Monolith Corpectomy 14mm 3 Level |
| 00887517706195 | Monolith Corpectomy 14mm Implant Tray |
| 00887517706188 | Monolith Corpectomy 12mm 3 Level |
| 00887517706171 | Monolith Corpectomy 12mm Implant Tray |
| 00887517698261 | Monolith Endcap, 19x16mm Lord 15° Ø14 |
| 00887517698254 | Monolith Endcap, 17x15mm Lord 15° Ø14 |
| 00887517698247 | Monolith Endcap, 17x14mm Lord 15° Ø12 |
| 00887517698230 | Monolith Endcap, 15x13mm Lord 15° Ø12 |
| 00887517698223 | Monolith Endcap, 22x19mm Lord 7° Ø14 |
| 00887517698216 | Monolith Endcap, 22x19mm Lord 15° Ø14 |
| 00887517698209 | Monolith Endcap, 22x19mm Lord 10° Ø14 |
| 00887517698087 | Monolith Core, Ø14x48mm |
| 00887517698070 | Monolith Core, Ø14x46mm |
| 00887517698063 | Monolith Core, Ø14x44mm |
| 00887517698056 | Monolith Core, Ø14x42mm |
| 00887517698049 | Monolith Core, Ø14x40mm |
| 00887517698032 | Monolith Core, Ø14x38mm |
| 00887517698025 | Monolith Core, Ø14x36mm |
| 00887517698018 | Monolith Core, Ø14x34mm |
| 00887517698001 | Monolith Core, Ø14x32mm |
| 00887517697998 | Monolith Core, Ø14x30mm |
| 00887517697981 | Monolith Core, Ø14x28mm |
| 00887517697974 | Monolith Core, Ø14x26mm |
| 00887517697967 | Monolith Core, Ø14x24mm |
| 00887517697950 | Monolith Core, Ø14x22mm |
| 00887517697943 | Monolith Core, Ø12x48mm |
| 00887517697936 | Monolith Core, Ø12x46mm |
| 00887517697929 | Monolith Core, Ø12x44mm |
| 00887517697912 | Monolith Core, Ø12x42mm |
| 00887517697905 | Monolith Core, Ø12x40mm |
| 00887517697899 | Monolith Core, Ø12x38mm |
| 00887517697882 | Monolith Core, Ø12x36mm |
| 00887517697875 | Monolith Core, Ø12x34mm |
| 00887517697868 | Monolith Core, Ø12x32mm |
| 00887517697851 | Monolith Core, Ø12x30mm |
| 00887517697844 | Monolith Core, Ø12x28mm |
| 00887517697837 | Monolith Core, Ø12x26mm |
| 00887517697820 | Monolith Core, Ø12x24mm |
| 00887517697813 | Monolith Core, Ø12x22mm |
| 00887517697806 | Monolith Endcap, 22x19mm Parallel Ø14 |
| 00887517677587 | Monolith Removal Tool, Endcap |
| 00887517677570 | Monolith Tamp |
| 00887517677563 | Monolith Positioning Tool, Implant |
| 00887517677518 | Monolith Loading Tool, Graft |
| 00887517677471 | Monolith Trial, Endcap Ø14/Ø16 |
| 00887517677464 | Monolith Trial, Endcap 17x15/19x16 |
| 00887517677457 | Monolith Trial, Endcap 15x13/17x14 |
Trademark Results [Monolith]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MONOLITH 98752663 not registered Live/Pending |
Omnitype Design LLC 2024-09-16 |
 MONOLITH 98712413 not registered Live/Pending |
Manamotley LLC 2024-08-22 |
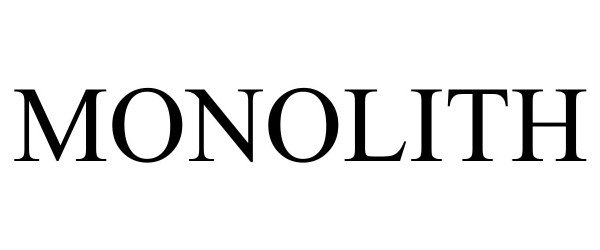 MONOLITH 98480294 not registered Live/Pending |
Spout Ventures Inc. 2024-04-02 |
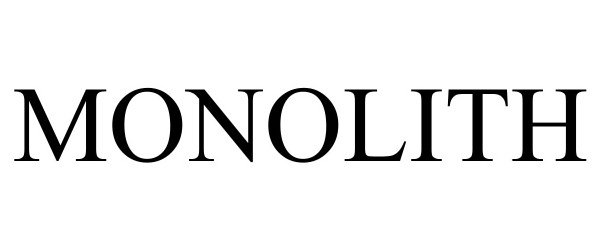 MONOLITH 98274997 not registered Live/Pending |
Monolith AI Limited 2023-11-17 |
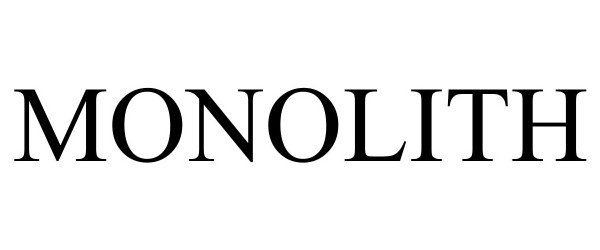 MONOLITH 97725911 not registered Live/Pending |
Newbasis, LLC 2022-12-20 |
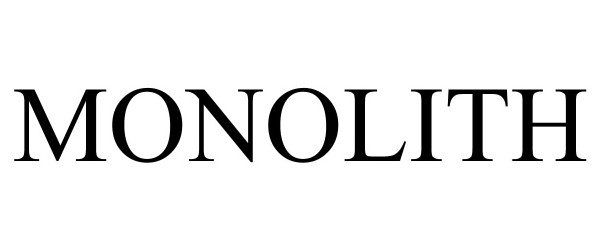 MONOLITH 97310383 not registered Live/Pending |
BASARIC, DENIS 2022-03-14 |
 MONOLITH 97217795 not registered Live/Pending |
Monoprice, Inc. 2022-01-13 |
 MONOLITH 90840568 not registered Live/Pending |
Monolith Materials, Inc. 2021-07-21 |
 MONOLITH 90681231 not registered Live/Pending |
Monolith LLC 2021-04-29 |
 MONOLITH 90163674 not registered Live/Pending |
Prisman Inc. 2020-09-07 |
 MONOLITH 88315536 not registered Live/Pending |
Conmed Corporation 2019-02-25 |
 MONOLITH 88286754 not registered Live/Pending |
Lian Yun Gang An Han E-commerce Ltd 2019-02-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.