ILIF
GUDID 00887517984920
RMM Navigation.S Instruments
Nuvasive, Inc.
Sterilization/disinfection container| Primary Device ID | 00887517984920 |
| NIH Device Record Key | f359875b-257a-4f80-bc06-d8505db6c923 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ILIF |
| Version Model Number | RMMNAVS |
| Company DUNS | 053950783 |
| Company Name | Nuvasive, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1(858)909-1800 |
| RA_UDI@nuvasive.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com | |
| Phone | +1800-475-9131 |
| nuvainfo@globusmedical.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00887517984920 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K143579
FDA Product Code
| KCT | Sterilization wrap containers, trays, cassettes & other accessories |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
[00887517984920]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2019-09-10 |
| Device Publish Date | 2019-01-04 |
On-Brand Devices [ILIF]
| 00887517519153 | Interspinous General Ins Set |
| 00887517515513 | Alpha H3 Trial Tray |
| 00887517263711 | ILIF Trial, 20mm |
| 00887517263704 | ILIF Trial, 18mm |
| 00887517263391 | ILIF Trial, 16mm |
| 00887517263384 | ILIF Trial, 14mm |
| 00887517263377 | ILIF Trial, 12mm |
| 00887517263360 | ILIF Trial, 10mm |
| 00887517263353 | ILIF Trial, 8mm |
| 00887517263339 | ILIF Tamp |
| 00887517263322 | ILIF Inserter, Tanged |
| 00887517262899 | ILIF Rack Distractor |
| 00887517262882 | ILIF Shovel Tip, Long |
| 00887517262868 | ILIF Distractor Tube |
| 00887517262851 | ILIF Shovel Tip |
| 00887517262837 | ILIF Driver, Caspar Pin |
| 00887517257529 | ILIF Rasp |
| 00887517257192 | ILIF Tissue Dissector |
| 00887517984920 | RMM Navigation.S Instruments |
| 00887517709493 | MAS Midline Trial, L |
| 00887517709486 | MAS Midline Trial, M |
| 00887517709479 | MAS Midline Trial, S |
| 00887517262813 | Tray - Interspinous Instruments (Base) |
Trademark Results [ILIF]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
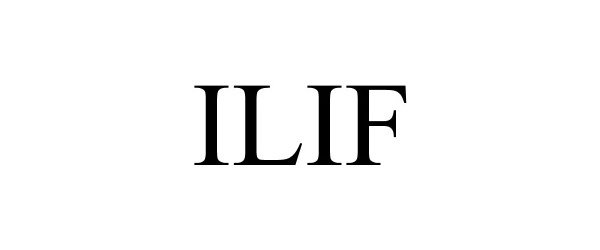 ILIF 90690844 not registered Live/Pending |
Globus Medical, Inc. 2021-05-05 |
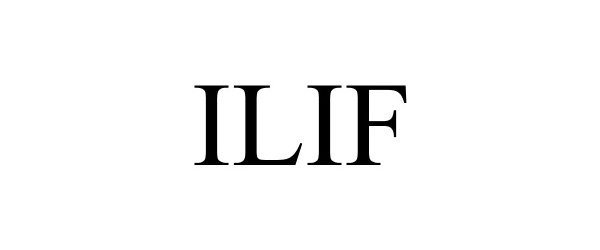 ILIF 85323114 4207111 Dead/Cancelled |
NuVasive Inc. 2011-05-17 |
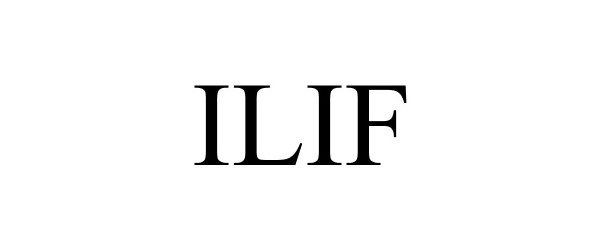 ILIF 77607150 not registered Dead/Abandoned |
NuVasive, Inc. 2008-11-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.