X-FIX 09590
GUDID 00887868084447
Biomet Orthopedics, LLC
External orthopaedic fixation system, single-use, non-sterile| Primary Device ID | 00887868084447 |
| NIH Device Record Key | 88a2d20a-edec-4ece-9b78-d9af01292a8b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | X-FIX |
| Version Model Number | 09590 |
| Catalog Number | 09590 |
| Company DUNS | 129278169 |
| Company Name | Biomet Orthopedics, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com | |
| Phone | +1(800)348-9500 |
| customerservice@zimmerbiomet.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00887868084447 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K020403
FDA Product Code
| KTT | APPLIANCE, FIXATION, NAIL/BLADE/PLATE COMBINATION, MULTIPLE COMPONENT |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
[00887868084447]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-10-28 |
Devices Manufactured by Biomet Orthopedics, LLC
| 00880304009615 - BMP CABLE CUTTER | 2026-02-11 |
| 00880304387676 - OPEN END CUTTER | 2026-02-11 |
| 00887868000027 - TWIST DRILL BIT | 2026-02-11 |
| 00887868000645 - CALIBRATED TWIST DRILL | 2026-02-11 |
| 00887868048265 - EASYOUT | 2026-02-11 |
| 00887868048319 - EXTRACTION BOLT | 2026-02-11 |
| 00887868048326 - EXTRACTION BOLT | 2026-02-11 |
| 00887868048333 - EXTRACTION BOLT | 2026-02-11 |
Trademark Results [X-FIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
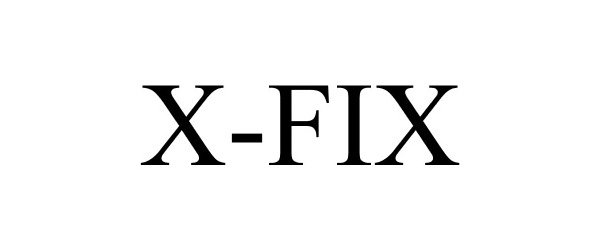 X-FIX 85860189 4800131 Live/Registered |
Jochen Klieber 2013-02-26 |
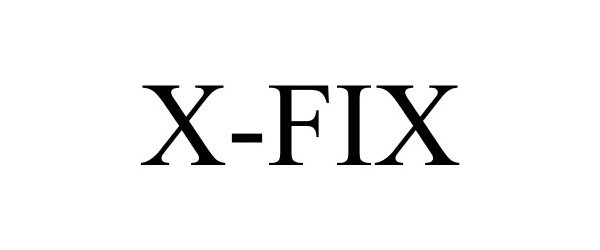 X-FIX 85691393 not registered Dead/Abandoned |
RX Devices, LLC 2012-07-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.