FORK
GUDID 00888118120144
KLS-Martin L.P.
Suturing unit, reusable| Primary Device ID | 00888118120144 |
| NIH Device Record Key | 27a37680-ad49-4f4b-abb3-6d1c9f5a57a4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | FORK |
| Version Model Number | 24-856-10-07 |
| Company DUNS | 826499238 |
| Company Name | KLS-Martin L.P. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00888118120144 [Primary] |
FDA Product Code
| HXE | FORK |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
[00888118120144]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-02-06 |
| Device Publish Date | 2018-11-22 |
On-Brand Devices [FORK]
| 00888118120144 | 24-856-10-07 |
| 00888118064400 | 38-885-18-07 |
Trademark Results [FORK]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
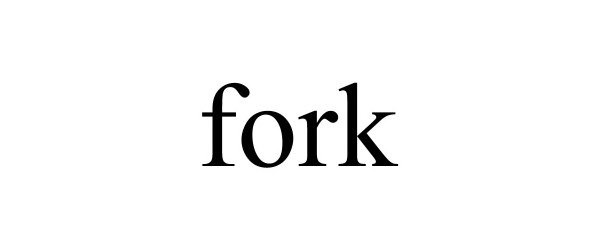 FORK 88843305 not registered Live/Pending |
Psyhogeos, Nick 2020-03-22 |
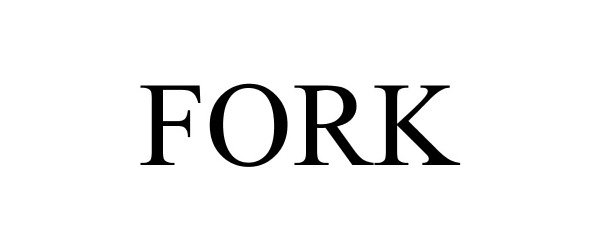 FORK 87763856 not registered Live/Pending |
Raffles Investments (Proprietary) Limited 2018-01-21 |
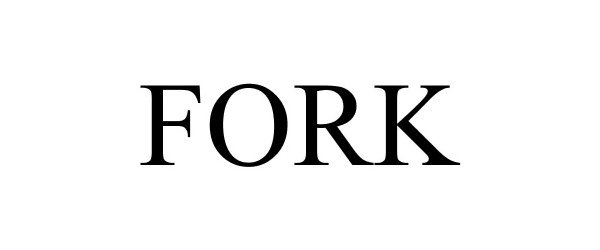 FORK 87584766 5618568 Live/Registered |
Bereber, Brian 2017-08-25 |
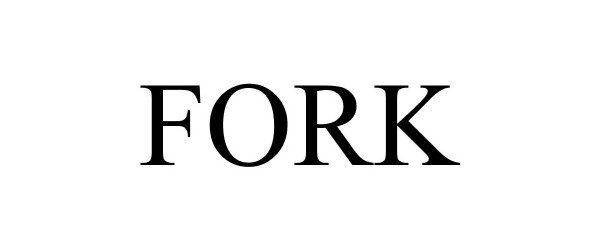 FORK 87472426 5522340 Live/Registered |
FORK INTERNATIONAL LIMITED 2017-06-01 |
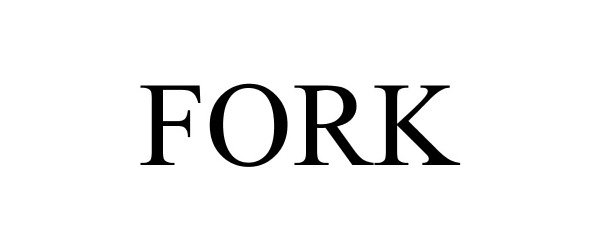 FORK 86178039 not registered Dead/Abandoned |
ADVANCE MAGAZINE PUBLISHERS INC. 2014-01-28 |
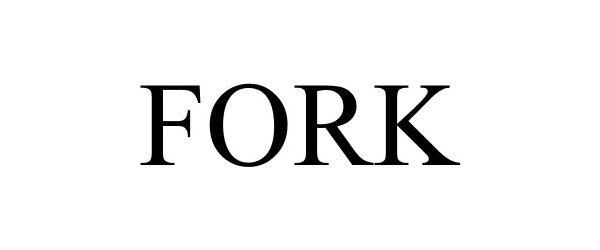 FORK 85110785 not registered Dead/Abandoned |
ADVANCE MAGAZINE PUBLISHERS INC. 2010-08-18 |
 FORK 78286689 not registered Dead/Abandoned |
River Road of Sag Harbor, NY 2003-08-13 |
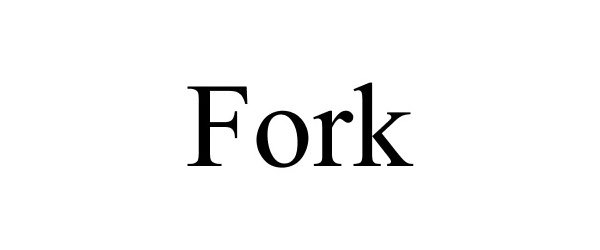 FORK 77784229 not registered Dead/Abandoned |
Mesh, Mikel 2009-07-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.