MediChoice
GUDID 00888937010312
MediChoice® 8 MHz Vascular Probe
Coopersurgical, Inc.
Noninvasive vascular ultrasound system probe| Primary Device ID | 00888937010312 |
| NIH Device Record Key | a5ec8aa9-27f9-4832-9baa-f497594ee8cd |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MediChoice |
| Version Model Number | MCSD8 |
| Company DUNS | 801895244 |
| Company Name | Coopersurgical, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00888937010312 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K024197
FDA Product Code
| HEP | Monitor, Blood-Flow, Ultrasonic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-23 |
On-Brand Devices [MediChoice]
| 60888937010697 | MediChoice® Vista AVS Advanced ABI Automated Vascular System |
| 60888937010260 | MediChoice® Handheld ABI Doppler System |
| 60888937010253 | MediChoice® Handheld ABI Doppler System |
| 00888937010688 | Display Handheld Doppler with 2 MHz Waterproof Obstetrical Probe (MC250SDW) |
| 00888937010671 | Display Handheld Doppler with 8 MHz Vascular Probe (MC250SD8) |
| 00888937010664 | Display Handheld Doppler with 5 MHz Vascular Probe (MC250SD5) |
| 00888937010657 | Display Handheld Doppler with 4 MHz Vascular Probe (MC250SD4) |
| 00888937010640 | Display Handheld Doppler with 3 MHz Obstetrical Probe (MC250SD3) |
| 00888937010633 | Display Handheld Doppler with 2 MHz Obstetrical Probe (MC250SD2) |
| 00888937010626 | Display Handheld Doppler with Recharger and 2 MHz Waterproof Obstetrical Probe (M250RSDW) |
| 00888937010619 | Display Handheld Doppler with Recharger and 8 MHz Vascular Probe (M250RSD8) |
| 00888937010602 | Display Handheld Doppler with Recharger and 5 MHz Vascular Probe (M250RSD5) |
| 00888937010596 | Display Handheld Doppler with Recharger and 4 MHz Vascular Probe (M250RSD4) |
| 00888937010589 | Display Handheld Doppler with Recharger and 3 MHz Obstetrical Probe (M250RSD3) |
| 00888937010572 | Display Handheld Doppler with Recharger and 2 MHz Obstetrical Probe (M250RSD2) |
| 00888937010565 | Display Handheld Doppler with Recharger, Audio Recorder and 2 MHz Waterproof Obstetrical Probe ( |
| 00888937010558 | Display Handheld Doppler with Recharger, Audio Recorder and 8 MHz Vascular Probe (250ARSD8) |
| 00888937010541 | Display Handheld Doppler with Recharger, Audio Recorder and 5 MHz Vascular Probe (250ARSD5) |
| 00888937010534 | Display Handheld Doppler with Recharger, Audio Recorder and 4 MHz Vascular Probe (250ARSD4) |
| 00888937010527 | Display Handheld Doppler with Recharger, Audio Recorder and 3 MHz Obstetrical Probe (250ARSD3) |
| 00888937010510 | Display Handheld Doppler with Recharger, Audio Recorder and 2 MHz Obstetrical Probe (250ARSD2) |
| 00888937010503 | Non-Display Handheld Doppler with 2 MHz Waterproof Obstetrical Probe (MC150SDW) |
| 00888937010497 | Non-Display Handheld Doppler with 8 MHz Vascular Probe (MC150SD8) |
| 00888937010480 | Non-Display Handheld Doppler with 5 MHz Vascular Probe (MC150SD5) |
| 00888937010473 | Non-Display Handheld Doppler with 4 MHz Vascular Probe (MC150SD4) |
| 00888937010466 | Non-Display Handheld Doppler with 3 MHz Obstetrical Probe (MC150SD3) |
| 00888937010459 | Non-Display Handheld Doppler with 2 MHz Obstetrical Probe (MC150SD) |
| 00888937010442 | Non-Display Handheld Doppler with Recharger and 2 MHz Waterproof Obstetrical Probe (M150RSDW) |
| 00888937010435 | Non-Display Handheld Doppler with Recharger and 8 MHz Vascular Probe (M150RSD8) |
| 00888937010428 | Non-Display Handheld Doppler with Recharger and 5 MHz Vascular Probe (M150RSD5) |
| 00888937010411 | Non-Display Handheld Doppler with Recharger and 4 MHz Vascular Probe (M150RSD4) |
| 00888937010404 | Non-Display Handheld Doppler with Recharger and 3 MHz Obstetrical Probe (M150RSD3) |
| 00888937010398 | Non-Display Handheld Doppler with Recharger and 2 MHz Obstetrical Probe (M150RSD2) |
| 00888937010381 | Non-Display Handheld Doppler with Audio Recorder and 2 MHz Waterproof Obstetrical Probe (M150ASD |
| 00888937010374 | Non-Display Handheld Doppler with Audio Recorder and 8 MHz Vascular Probe (M150ASD8) |
| 00888937010350 | Non-Display Handheld Doppler with Audio Recorder and 4 MHz Vascular Probe (M150ASD4) |
| 00888937010343 | Non-Display Handheld Doppler with Audio Recorder and 3 MHz Obstetrical Probe (M150ASD3) |
| 00888937010336 | Non-Display Handheld Doppler with Audio recorder and 2 MHz Obstetrical Probe (M150ASD2) |
| 00888937010329 | MediChoice® 2 MHz Obstetrical Waterproof Probe |
| 00888937010312 | MediChoice® 8 MHz Vascular Probe |
| 00888937010305 | MediChoice® 5 MHz Vascular Probe |
| 00888937010299 | MediChoice® 4 MHz Vascular Probe |
| 00888937010282 | MediChoice® 3 MHz Obstetrical Probe |
| 00888937010275 | MediChoice® 2 MHz Obstetrical Probe |
| 00888937010244 | MediChoice® Display Tabletop Doppler With Recharger And 2 MHz Waterproof Obstetrical Probe |
| 00888937010237 | MediChoice® Display Tabletop Doppler With Recharger And 8 MHz Vascular Probe |
| 00888937010220 | MediChoice® Display Tabletop Doppler With Recharger And 5 MHz Vascular Probe |
| 00888937010213 | MediChoice® Display Tabletop Doppler With Recharger And 4 MHz Vascular Probe |
| 00888937010206 | MediChoice® Display Tabletop Doppler With Recharger And 3 MHz Obstetrical Probe |
| 00888937010190 | MediChoice® Display Tabletop Doppler With Recharger And 2 MHz Obstetrical Probe |
Trademark Results [MediChoice]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEDICHOICE 78570543 not registered Dead/Abandoned |
Mount Carmel Health Plan, Inc. 2005-02-18 |
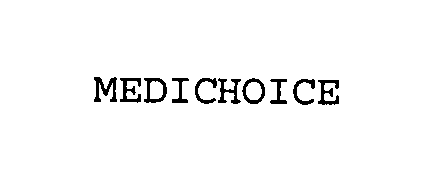 MEDICHOICE 76978548 3283434 Live/Registered |
Owens & Minor, Inc. 2001-12-26 |
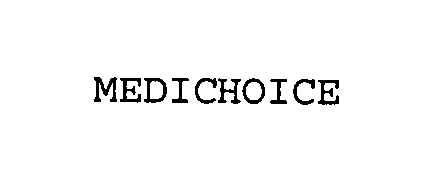 MEDICHOICE 76352417 3462755 Live/Registered |
Owens & Minor, Inc. 2001-12-26 |
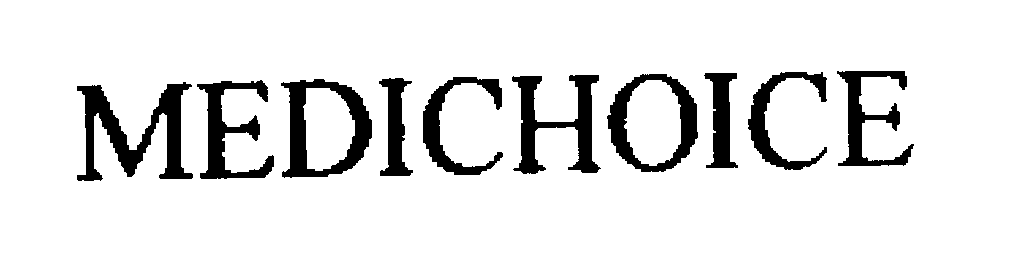 MEDICHOICE 76285309 2753896 Live/Registered |
Owens & Minor, Inc. 2001-07-13 |
 MEDICHOICE 75267174 not registered Dead/Abandoned |
Blue Ridge Health Alliance 1997-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.