Hex-Lock®
GUDID 00889024015227
ZIMMER DENTAL INC.
Dental implant suprastructure, permanent, preformed| Primary Device ID | 00889024015227 |
| NIH Device Record Key | 67466eed-9067-49fb-a85a-89bbe13aab7e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Hex-Lock® |
| Version Model Number | HLA4/6 |
| Company DUNS | 103167086 |
| Company Name | ZIMMER DENTAL INC. |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00889024015227 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K011028
FDA Product Code
| DZE | IMPLANT, ENDOSSEOUS, ROOT-FORM |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 5 |
| Public Version Date | 2019-11-08 |
| Device Publish Date | 2015-10-09 |
On-Brand Devices [Hex-Lock® ]
| 00889024020900 | ZOA563S |
| 00889024020894 | ZOA562S |
| 00889024020887 | ZOA562A |
| 00889024020870 | ZOA561S |
| 00889024020863 | ZOA561A |
| 00889024020801 | ZOA443S |
| 00889024020795 | ZOA442S |
| 00889024020788 | ZOA442A |
| 00889024020771 | ZOA441S |
| 00889024020764 | ZOA441A |
| 00889024020757 | ZOA343S |
| 00889024020740 | ZOA342S |
| 00889024020733 | ZOA342A |
| 00889024020726 | ZOA341S |
| 00889024020719 | ZOA341A |
| 00889024016798 | SA562 |
| 00889024016781 | SA561 |
| 00889024016774 | SA452 |
| 00889024016767 | SA451 |
| 00889024016750 | SA342 |
| 00889024016743 | SA341 |
| 00889024015241 | HLA5/6 |
| 00889024015227 | HLA4/6 |
| 00889024015210 | HLA4/5 |
| 00889024015203 | HLA4/4 |
| 00889024015180 | HLA3/5 |
| 00889024015173 | HLA3/4L |
| 00889024015166 | HLA3/4 |
| 00889024015159 | HLA3/3 |
| 00889024015142 | HLA2F |
Trademark Results [Hex-Lock]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
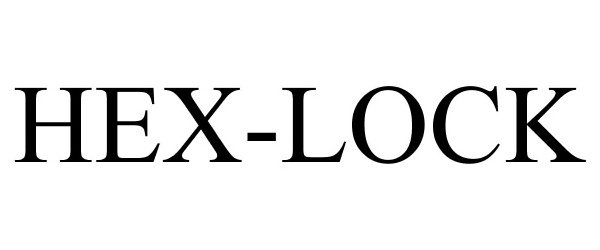 HEX-LOCK 78921253 3237289 Live/Registered |
Zimmer Dental Inc. 2006-06-30 |
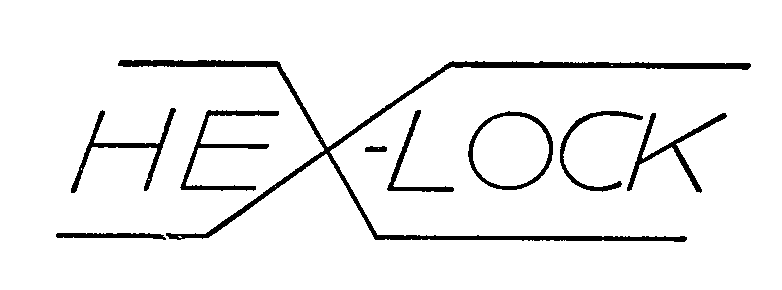 HEX-LOCK 72149319 0758754 Dead/Expired |
PLAS-STEEL PRODUCTS, INC. 1962-07-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.