Nexel™ Vivacit-E® 00-8400-095-00
GUDID 00889024271463
Zimmer, Inc.
Hinged total elbow prosthesis| Primary Device ID | 00889024271463 |
| NIH Device Record Key | 702c2230-ae77-462c-90eb-67853d2c6efd |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Nexel™ Vivacit-E® |
| Version Model Number | 00-8400-095-00 |
| Catalog Number | 00-8400-095-00 |
| Company DUNS | 056038268 |
| Company Name | Zimmer, Inc. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00889024271463 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K123862
FDA Product Code
| JDC | PROSTHESIS, ELBOW, CONSTRAINED, CEMENTED |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2019-11-06 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [Nexel™ Vivacit-E®]
| 00889024271463 | 00-8400-095-00 |
| 00889024271456 | 00-8400-094-00 |
Trademark Results [Nexel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NEXEL 90254764 not registered Live/Pending |
Nexel Technologies Corporation 2020-10-14 |
 NEXEL 90141339 not registered Live/Pending |
NEXEL TECHNOLOGIES CORPORATION 2020-08-27 |
 NEXEL 88024092 not registered Live/Pending |
Kingsberg, George 2018-07-03 |
 NEXEL 87979048 5634674 Live/Registered |
Nexel Industries, Inc. 2016-11-03 |
 NEXEL 87975692 5293001 Live/Registered |
Nexel Industries, Inc. 2016-11-03 |
 NEXEL 87709026 5513060 Live/Registered |
Nexel Industries, Inc. 2017-12-05 |
 NEXEL 87495635 5419950 Live/Registered |
Nexel Industries, Inc. 2017-06-19 |
 NEXEL 87225437 not registered Live/Pending |
Nexel Industries, Inc. 2016-11-03 |
 NEXEL 86887318 5031814 Live/Registered |
Nexel Industries, Inc. 2016-01-26 |
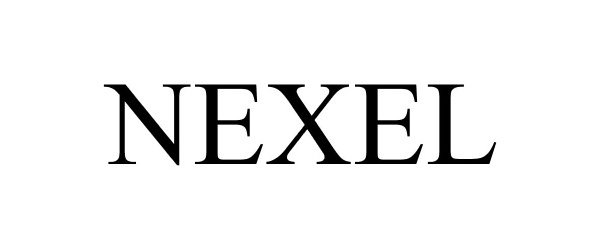 NEXEL 85730768 4433700 Live/Registered |
Zimmer, Inc. 2012-09-17 |
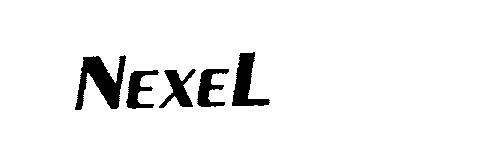 NEXEL 74493288 not registered Dead/Abandoned |
Accelerated Systems, Inc. 1994-02-22 |
 NEXEL 74059539 1717888 Live/Registered |
Global Equipment Company 1990-05-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.