TraXis®
GUDID 00889024332966
ZIMMER SPINE, INC.
Metallic spinal fusion cage, non-sterile| Primary Device ID | 00889024332966 |
| NIH Device Record Key | 3ec53a70-2f21-43b5-8db6-e0a4048394f2 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | TraXis® |
| Version Model Number | 2602-090925 |
| Company DUNS | 787663400 |
| Company Name | ZIMMER SPINE, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
| Width | 9 Millimeter |
| Height | 9 Millimeter |
| Length | 25 Millimeter |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00889024332966 [Primary] |
FDA Product Code
| MQP | SPINAL VERTEBRAL BODY REPLACEMENT DEVICE |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
[00889024332966]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [TraXis®]
| 00889024332942 | 2601-151125 |
| 00889024332935 | 2601-151121 |
| 00889024332928 | 2601-131125 |
| 00889024332911 | 2601-131121 |
| 00889024332904 | 2601-130925 |
| 00889024332898 | 2601-130921 |
| 00889024332881 | 2601-111125 |
| 00889024332874 | 2601-111121 |
| 00889024332867 | 2601-110925 |
| 00889024332850 | 2601-110921 |
| 00889024332843 | 2601-090925 |
| 00889024332836 | 2601-090921 |
| 00889024332829 | 2601-070925 |
| 00889024332812 | 2601-070921 |
| 00889024333062 | 2602-151125 |
| 00889024333055 | 2602-151121 |
| 00889024333048 | 2602-131125 |
| 00889024333031 | 2602-131121 |
| 00889024333000 | 2602-111125 |
| 00889024332997 | 2602-111121 |
| 00889024332980 | 2602-110925 |
| 00889024332973 | 2602-110921 |
| 00889024332966 | 2602-090925 |
| 00889024332959 | 2602-090921 |
Trademark Results [TraXis]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
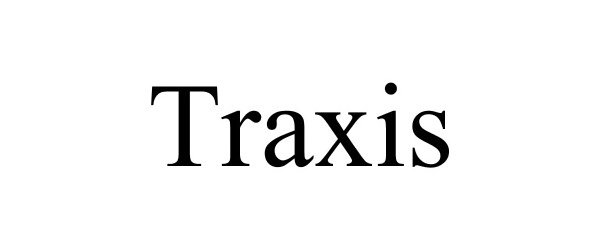 TRAXIS 85175649 3978167 Dead/Cancelled |
TLH, Inc. 2010-11-12 |
 TRAXIS 79332992 not registered Live/Pending |
ZERODENSITY YAZILIM ANONIM SIRKETI 2021-12-01 |
 TRAXIS 78197286 2949816 Live/Registered |
ZIMMER SPINE, INC. 2002-12-23 |
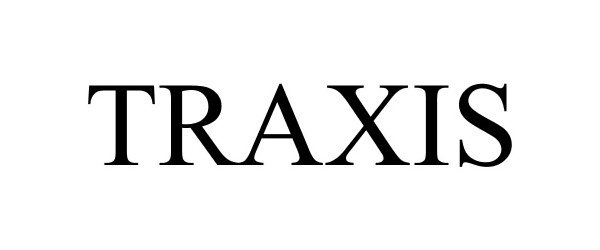 TRAXIS 77275559 3554923 Dead/Cancelled |
National Marrow Donor Program 2007-09-10 |
 TRAXIS 76403655 not registered Dead/Abandoned |
Eastech Flow Controls, Inc. 2002-05-03 |
 TRAXIS 76338565 not registered Dead/Abandoned |
COBRA ELECTRONICS CORPORATION 2001-11-15 |
 TRAXIS 75699298 2828763 Dead/Cancelled |
Micronic B.V. 1999-05-07 |
 TRAXIS 75360618 2231684 Dead/Cancelled |
TRAXIS INC. 1997-09-22 |
 TRAXIS 73685527 1520621 Dead/Cancelled |
TRAXIS INC. 1987-09-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.