Optium® TGEL10
GUDID 00889858450584
Optium DBM Gel
LIFENET HEALTH
Bone matrix implant, human-derived| Primary Device ID | 00889858450584 |
| NIH Device Record Key | 3b61944d-934f-4d45-b1d1-24e27b9f9ba6 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Optium® |
| Version Model Number | TGEL10 |
| Catalog Number | TGEL10 |
| Company DUNS | 121828156 |
| Company Name | LIFENET HEALTH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | true |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx | |
| Phone | 1-888-847-7831 |
| xx@xx.xx |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 00889858450584 [Primary] |
FDA Product Code
| MQV | Filler, Bone Void, Calcium Compound |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2020-12-10 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [Optium®]
| 00889858517744 | Optium DBM Putty |
| 00889858517720 | Optium DBM Putty |
| 00889858517706 | Optium DBM Putty |
| 00889858517683 | Optium DBM Putty |
| 00889858450584 | Optium DBM Gel |
| 00889858450560 | Optium DBM Gel |
| 00889858450546 | Optium DBM Gel |
| 00889858625708 | Optium DBM Gel, 3 cc |
Trademark Results [Optium]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OPTIUM 85733300 4334589 Live/Registered |
LifeNet Health 2012-09-19 |
 OPTIUM 85197960 not registered Dead/Abandoned |
Cardium Therapeutics, Inc. 2010-12-15 |
 OPTIUM 78546826 3041682 Live/Registered |
Tru Vue, Inc. 2005-01-13 |
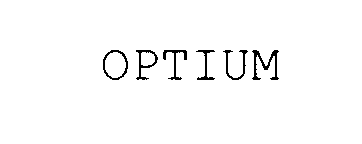 OPTIUM 78036804 2596074 Live/Registered |
Veeco Instruments Inc. 2000-11-28 |
 OPTIUM 77560052 3602208 Dead/Cancelled |
GF Health Products, Inc. 2008-09-02 |
 OPTIUM 76162743 not registered Dead/Abandoned |
DERMISPA GROUP, INC. 2000-11-09 |
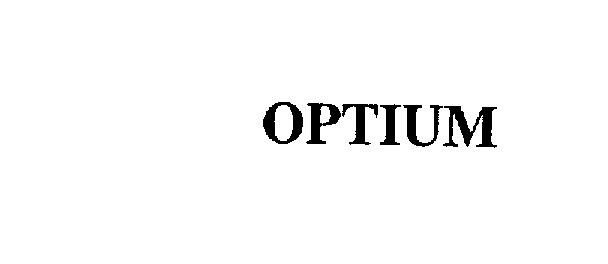 OPTIUM 75514378 2402538 Dead/Cancelled |
TRU VUE, INC. 1998-07-06 |
 OPTIUM 75514326 not registered Dead/Abandoned |
VIRATEC THIN FILMS, INC. 1998-07-06 |
 OPTIUM 75119354 not registered Dead/Abandoned |
TEXAS INSTRUMENTS INCORPORATED 1996-06-14 |
 OPTIUM 74672228 1988291 Dead/Cancelled |
Fay's Incorporated 1995-05-11 |
 OPTIUM 74529673 2120685 Dead/Cancelled |
PROMAU S.R.L. 1994-05-26 |
 OPTIUM 74291384 not registered Dead/Abandoned |
Intel Corporation 1992-07-02 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.