EMAG® SOFTWARE V1.4
GUDID 03573026635046
EMAG software (virtual) version 1.4 also called SP4
BIOMERIEUX SA
Nucleic acid sample preparation instrument IVD| Primary Device ID | 03573026635046 |
| NIH Device Record Key | c0645d3c-945e-4027-9739-ff53c0dddda4 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | EMAG® SOFTWARE V1.4 |
| Version Model Number | 1.4 |
| Company DUNS | 276816717 |
| Company Name | BIOMERIEUX SA |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03573026635046 [Primary] |
FDA Product Code
| JJH | Clinical sample concentrator |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-02-12 |
| Device Publish Date | 2024-02-02 |
Devices Manufactured by BIOMERIEUX SA
| 03573026644796 - VIDAS® Varicella-Zoster IgG | 2026-01-27 VIDAS VARICEL. ZOSTER IGG assay is a qualitative IA intended for use on VIDAS family instrument for the detection of IgG antibod |
| 03573026092009 - ID color Catalase | 2026-01-23 Manual reagent for catalase test. API®strips give accurate identifications based on extensive databases and are standardized, e |
| 03573026216801 - CHROMID® P. aeruginosa Agar | 2026-01-21 Direct identification of Pseudomonas aeruginosa in pulmonary specimens. |
| 03573026118952 - CHROMID® Candida Agar | 2026-01-21 Chromogenic medium for the selective isolation of yeasts and the direct identification of Candida albicans |
| 03573026316969 - CHROMID® C. difficile | 2026-01-21 Chromogenic medium for the detection and ident ification of Clostridium difficile. |
| 03573026374846 - CHROMID® Salmonella Elite | 2026-01-19 chromID™ Salmonella Eliteagar is a chromogenic medium for the selective isolation and identification of Salmonellain human spe |
| 03573026443900 - CHROMID® CPS® Elite Agar (CPSE) | 2026-01-19 Isolation, enumeration and direct or presumptive identification of urinary tract infection organisms |
| 03573026468170 - CHROMID® CPS® Elite Agar (CPSO) | 2026-01-19 Isolation, enumeration and direct or presumptive identification of urinary tract infection organisms |
Trademark Results [EMAG]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
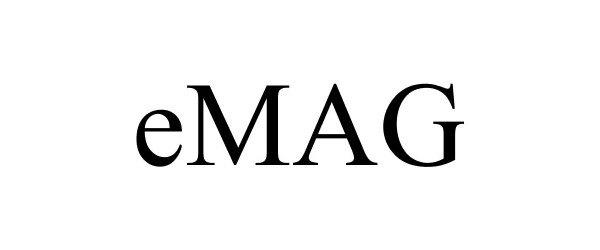 EMAG 87794123 not registered Live/Pending |
Ampere Computing LLC 2018-02-12 |
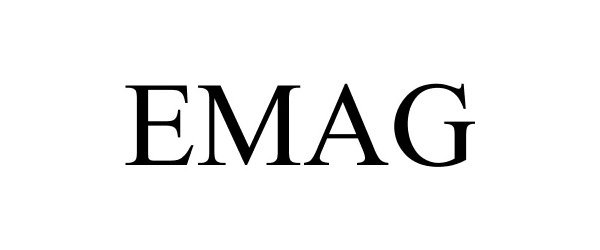 EMAG 86196703 4609346 Live/Registered |
Van Eck Associates Corporation 2014-02-18 |
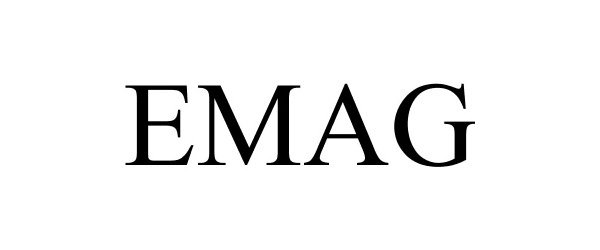 EMAG 85385069 4228693 Live/Registered |
Magpul Industries Corp. 2011-07-29 |
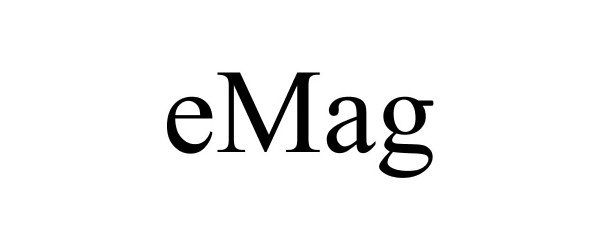 EMAG 85051435 not registered Dead/Abandoned |
Interweave Press, LLC 2010-06-01 |
 EMAG 79399107 not registered Live/Pending |
EMAG GmbH & Co. KG 2024-01-23 |
 EMAG 79176326 5056762 Live/Registered |
bioMérieux, Inc. 2015-08-06 |
 EMAG 79042654 not registered Dead/Abandoned |
HUGO BOSS Trade Mark Management GmbH & Co. KG 2007-05-29 |
 EMAG 78975722 not registered Dead/Abandoned |
CINE CRAFT LIMITED 2002-12-31 |
 EMAG 78198866 not registered Dead/Abandoned |
CINE CRAFT LIMITED 2002-12-31 |
 EMAG 76100731 not registered Dead/Abandoned |
Haiman International Industries, Incorporated 2000-08-01 |
 EMAG 75891119 2480865 Dead/Cancelled |
Airgun Designs, Inc. 2000-01-07 |
 EMAG 75791322 not registered Dead/Abandoned |
eMag Solutions LLC 1999-09-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.