Cytopro® Rotor
GUDID 03661540301179
The Cytopro Cytocentrifuge rotor is an in vitro diagnostic medical device for fixing biological cell suspensions on glass microscope slides for cytological examination. It is an accessory for use with any Elitech Group Aerospray Slide Stainer/Cytocentrifuge. This manual pertains only to the Series 2 stainer/cytocentrifuge models.
ELITECHGROUP INC.
Centrifugal sample concentrator| Primary Device ID | 03661540301179 |
| NIH Device Record Key | a33f1391-947c-4f49-aa53-68ef11cf49d3 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Cytopro® Rotor |
| Version Model Number | AC-160 |
| Company DUNS | 052423787 |
| Company Name | ELITECHGROUP INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com | |
| Phone | 1-800-453-2725 |
| service_ebs@elitechgoup.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03661540301179 [Primary] |
FDA Product Code
| IFB | Cytocentrifuge |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-03-01 |
| Device Publish Date | 2021-02-19 |
Devices Manufactured by ELITECHGROUP INC.
| 03661540300400 - Macroduct Advanced Sweat Collection System | 2025-03-07 The Macroduct Advanced Sweat Collection System is intended only for clinical laboratory use by qualified medical personnel for s |
| 03661540300035 - Microsed-System® Automated ESR Analyzer | 2023-10-09 The ESR analyzer is an automated, microprocessor controlled, in vitro diagnostic device that is intended for the quantitative de |
| 03661540300042 - Mix-Rate® X20 Automated ESR Analyzer | 2023-10-09 The ESR analyzer is an automated, microprocessor controlled, in vitro diagnostic device that is intended for the quantitative de |
| 03661540300202 - Monosed® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300219 - Monosed® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300240 - Excyte® ESR Non-Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300257 - Excyte® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300264 - Excyte® ESR Vacuum Tube, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
Trademark Results [Cytopro]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYTOPRO 97401623 not registered Live/Pending |
Marizyme, Inc. 2022-05-09 |
 CYTOPRO 85074461 not registered Dead/Abandoned |
CytoPRO, LLC 2010-06-30 |
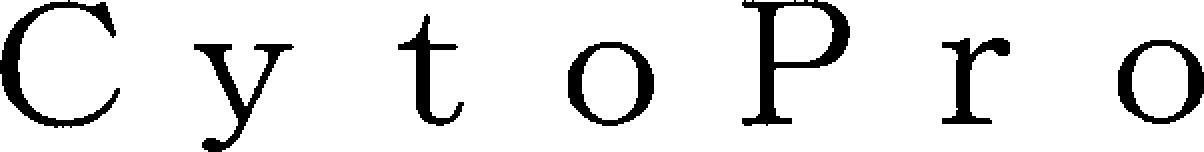 CYTOPRO 79243240 5859430 Live/Registered |
Pilica International Japan, Inc. 2018-06-08 |
 CYTOPRO 76649272 3147379 Dead/Cancelled |
Midwest Institute for Medical Education, Inc. 2005-10-21 |
 CYTOPRO 76319701 not registered Dead/Abandoned |
Theoharides, Dr. Theoharis C. 2001-09-28 |
 CYTOPRO 74713296 1982673 Dead/Cancelled |
Achievers Unlimited, Inc. 1995-08-09 |
 CYTOPRO 74284180 1839877 Live/Registered |
ELITECHGROUP INC. 1992-06-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.