Vapro® Vapor Pressure Osmometer
GUDID 03661540303425
The Vapro is a bench-top in-vitro diagnostic laboratory instrument for use by trained technical personnel to determine the osmolality concentration of a water based solution by means of the dew point temperature depression of a specimen sample.
ELITECHGROUP INC.
Osmometric analyser IVD, automated| Primary Device ID | 03661540303425 |
| NIH Device Record Key | 534c9298-3a53-4858-85de-177b99cdbfbb |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Vapro® Vapor Pressure Osmometer |
| Version Model Number | Model 5600 |
| Company DUNS | 052423787 |
| Company Name | ELITECHGROUP INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03661540303425 [Primary] |
FDA Product Code
| JNA | Vapor Pressure, Osmolality Of Serum & Urine |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2021-03-01 |
| Device Publish Date | 2021-02-19 |
Devices Manufactured by ELITECHGROUP INC.
| 03661540300400 - Macroduct Advanced Sweat Collection System | 2025-03-07 The Macroduct Advanced Sweat Collection System is intended only for clinical laboratory use by qualified medical personnel for s |
| 03661540300035 - Microsed-System® Automated ESR Analyzer | 2023-10-09 The ESR analyzer is an automated, microprocessor controlled, in vitro diagnostic device that is intended for the quantitative de |
| 03661540300042 - Mix-Rate® X20 Automated ESR Analyzer | 2023-10-09 The ESR analyzer is an automated, microprocessor controlled, in vitro diagnostic device that is intended for the quantitative de |
| 03661540300202 - Monosed® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300219 - Monosed® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300240 - Excyte® ESR Non-Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300257 - Excyte® ESR Vacuum Tubes, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
| 03661540300264 - Excyte® ESR Vacuum Tube, Carton | 2023-10-09 ESR Tubes are single use devices intended to be used as whole blood sample tubes for the quantitative determination of Erythrocy |
Trademark Results [Vapro]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
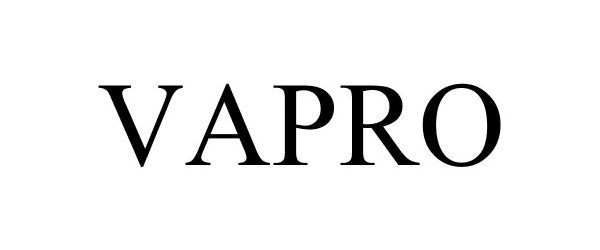 VAPRO 86780397 4964420 Live/Registered |
VAPRO Supply LLC 2015-10-07 |
 VAPRO 86779537 4964375 Live/Registered |
Vapro Supply LLC 2015-10-06 |
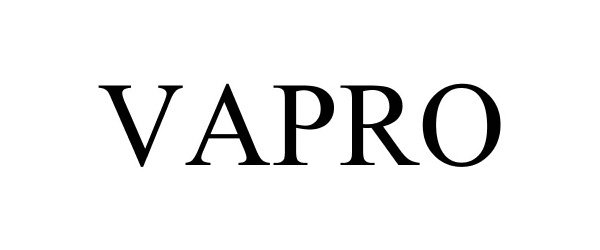 VAPRO 86015810 not registered Dead/Abandoned |
Vapro USA, LLC 2013-07-22 |
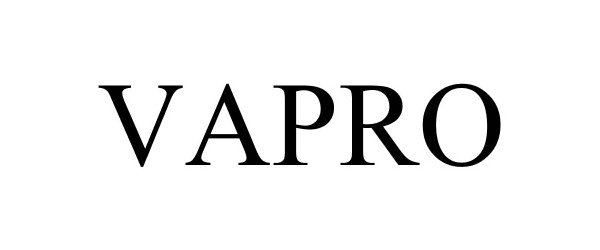 VAPRO 85922060 4549012 Live/Registered |
Hollister Incorporated 2013-05-02 |
 VAPRO 77814487 not registered Dead/Abandoned |
Hollister Incorporated 2009-08-27 |
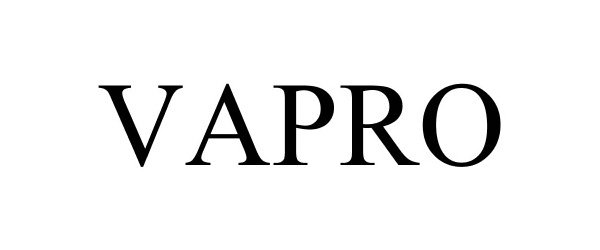 VAPRO 77051253 not registered Dead/Abandoned |
Hollister Incorporated 2006-11-27 |
 VAPRO 74567044 2001850 Live/Registered |
ELITECHGROUP INC. 1994-08-29 |
 VAPRO 74388418 1871942 Live/Registered |
SOUTHERN MILLS, INC. 1993-05-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.