Magic Needle
GUDID 03760264550204
NEEDLE CONCEPT
Hypodermic needle, single-use| Primary Device ID | 03760264550204 |
| NIH Device Record Key | 2c276b50-709f-41e0-a9fa-276efeca44ef |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Magic Needle |
| Version Model Number | MN25G40/23G |
| Company DUNS | 260304984 |
| Company Name | NEEDLE CONCEPT |
| Device Count | 50 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 03760264550044 [Unit of Use] |
| GS1 | 03760264550204 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K110606
FDA Product Code
| FMI | Needle, Hypodermic, Single Lumen |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2019-10-23 |
| Device Publish Date | 2019-04-05 |
On-Brand Devices [Magic Needle ]
| 03760264550235 | MN22G57/21G |
| 03760264550228 | MN22G70/21G |
| 03760264550211 | MN25G40/23G |
| 03760264550204 | MN25G40/23G |
| 03760264550198 | MN25G50/23G |
| 03760264550181 | MN27G37/26G |
| 03760264550174 | MN27G37/26G |
| 03760264550013 | MN30G27/27G |
Trademark Results [Magic Needle]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MAGIC NEEDLE 86892089 not registered Dead/Abandoned |
Robinson, Carol 2016-01-30 |
 MAGIC NEEDLE 86892089 not registered Dead/Abandoned |
Grumbles, Desiree 2016-01-30 |
 MAGIC NEEDLE 85572904 4271065 Live/Registered |
NEEDLE CONCEPT SAS 2012-03-19 |
 MAGIC NEEDLE 79137872 4622962 Live/Registered |
NEEDLE CONCEPT 2013-08-27 |
 MAGIC NEEDLE 74112873 1760634 Dead/Cancelled |
BRAIN LUCK DEVELOPMENT LIMITED 1990-11-06 |
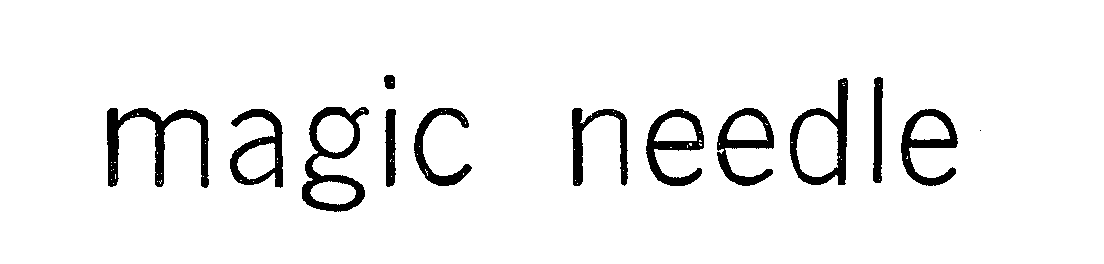 MAGIC NEEDLE 72186210 0780396 Dead/Expired |
VARSITY PAJAMAS, INCORPORATED 1964-02-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.