VOLTA 384075
GUDID 04035479143374
BIOTRONIK SE & Co. KG
Endocardial defibrillation lead| Primary Device ID | 04035479143374 |
| NIH Device Record Key | 425814cc-d71a-4ff2-8b2f-c430a1b5e8b1 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | VOLTA |
| Version Model Number | 1CR 65 |
| Catalog Number | 384075 |
| Company DUNS | 315620229 |
| Company Name | BIOTRONIK SE & Co. KG |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com | |
| Phone | +1(888)345-0374 |
| jon.brumbaugh@biotronik.com |
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store at temperatures up to 77°F; excursions permitted from 41°F to 131°F. |
| Storage Environment Temperature | Between 0 Degrees Fahrenheit and 77 Degrees Fahrenheit |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04035479143374 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P980023
FDA Product Code
| NVY | Permanent defibrillator electrodes |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2021-02-05 |
| Device Publish Date | 2014-08-19 |
On-Brand Devices [VOLTA]
| 04035479143435 | 2CT 65/18 |
| 04035479143428 | 2CR 75/18 |
| 04035479143411 | 2CR 65/18 |
| 04035479143404 | 2CR 65/16 |
| 04035479143398 | 2CR 60/16 |
| 04035479143381 | 1CR 75 |
| 04035479143374 | 1CR 65 |
| 04035479143367 | 1CR 60 |
| 04035479143145 | 2CT 65/16 |
| 04035479143138 | 1CT 65 |
| 04035479143121 | 2CT 75/18 |
Trademark Results [VOLTA]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VOLTA 98645932 not registered Live/Pending |
Volta Labs, Inc. 2024-07-12 |
 VOLTA 98635580 not registered Live/Pending |
Garcia, Emilio J 2024-07-06 |
 VOLTA 98594652 not registered Live/Pending |
Hydril USA Distribution LLC 2024-06-11 |
 VOLTA 98567241 not registered Live/Pending |
Volta Talent Strategies LLC 2024-05-24 |
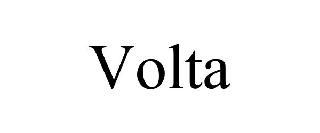 VOLTA 98562112 not registered Live/Pending |
Tech Quick Repair Inc 2024-05-21 |
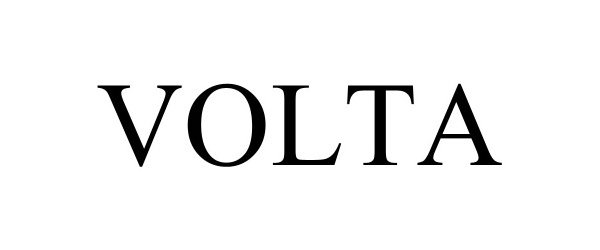 VOLTA 98533760 not registered Live/Pending |
Lumencor, Inc. 2024-05-03 |
 VOLTA 98474880 not registered Live/Pending |
Preston, Torri R 2024-03-29 |
 VOLTA 98474880 not registered Live/Pending |
Reed, Gordon F 2024-03-29 |
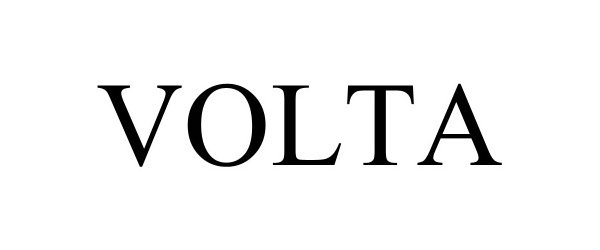 VOLTA 98438700 not registered Live/Pending |
NuKey, Inc. 2024-03-07 |
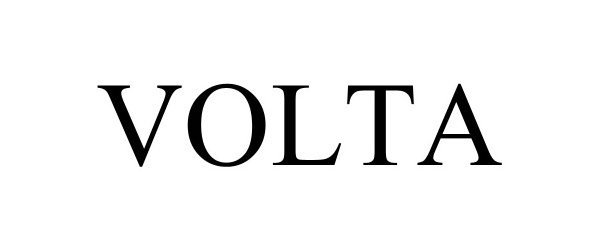 VOLTA 98124424 not registered Live/Pending |
Spark Biomedical, Inc. 2023-08-09 |
 VOLTA 97779812 not registered Live/Pending |
Io Financial Inc. 2023-02-03 |
 VOLTA 97768107 not registered Live/Pending |
VOLTA ROBOTS S.R.L. 2023-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.