Design Options® 560655
GUDID 04046955056941
SSK CENTRAL ARIZONA PAIN INSTITUTE
B. BRAUN MEDICAL INC.
Intrathecal anaesthesia set, non-medicated| Primary Device ID | 04046955056941 |
| NIH Device Record Key | ee053739-4b1a-4759-a88a-68c1a40bda17 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Design Options® |
| Version Model Number | 560655 |
| Catalog Number | 560655 |
| Company DUNS | 002397347 |
| Company Name | B. BRAUN MEDICAL INC. |
| Device Count | 10 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | true |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | true |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Operating and Storage Conditions
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
| Storage Environment Temperature | Between 20 Degrees Celsius and 25 Degrees Celsius |
| Handling Environment Temperature | Between 1 Degrees Celsius and 40 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04046955056934 [Unit of Use] |
| GS1 | 04046955056941 [Primary] |
FDA Product Code
| OFU | Spinal anesthesia kit |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2020-03-03 |
| Device Publish Date | 2019-02-02 |
On-Brand Devices [Design Options®]
| 04046955331741 | CESK-SD COMFORT SURGERY CENTER |
| 04046955290932 | BPSK COMFORT SURG CTR-NERVE BLOCK TRAY |
| 04046964808920 | Combined Spinal/Epidural Tray |
| 04046964321511 | Spinal Tray |
| 04046955324330 | CESK-SD CENTRAL ARIZONA PAIN INSTITUTE |
| 04046955290895 | BPSK MAIMONIDES HOSPITAL - NERVE BLOCK |
| 04046955290819 | CESK-SD BROADLAWNS MEDICAL CENTER |
| 04046955290796 | MEMORIAL HOSPITAL CESK-SD |
| 04046955184187 | CESK-SD UC HEALTH PAIN MEDICINE CENTER |
| 04046955056965 | SSK STONY BROOK HOSPITAL |
| 04046955056941 | SSK CENTRAL ARIZONA PAIN INSTITUTE |
| 04046955156528 | LYNDON B JOHNSON HOSPITAL SPINAL EPID |
| 04046964989926 | Continuous Epidural Tray |
| 04046964989902 | Continuous Epidural Tray |
| 04046964989780 | Continuous Epidural Tray |
| 04046964989766 | Continuous Epidural Tray |
| 04046964989742 | Continuous Epidural Tray |
| 04046964950452 | Combined Spinal/Epidural Tray |
| 04046964950438 | Combined Spinal/Epidural Tray |
| 04046964950414 | Combined Spinal/Epidural Tray |
| 04046964950391 | Combined Spinal/Epidural Tray |
| 04046964950377 | Combined Spinal/Epidural Tray |
| 04046964950353 | Single Dose Epidural Tray |
| 04046964950339 | Single Dose Epidural Tray |
| 04046964950315 | Single Dose Epidural Tray |
| 04046964950292 | Single Dose Epidural Tray |
| 04046964950278 | Single Dose Epidural Tray |
| 04046964950254 | Continuous Epidural Tray |
| 04046964950230 | Continuous Epidural Tray |
| 04046964950216 | Continuous Epidural Tray |
| 04046964950193 | Continuous Epidural Tray |
| 04046964950179 | Continuous Epidural Tray |
| 04046964910968 | Combined Spinal/Epidural Tray |
| 04046964910944 | Combined Spinal/Epidural Tray |
| 04046964848988 | Continuous Epidural Tray |
| 04046964843921 | Single Dose Epidural Tray |
| 04046964843907 | Single Dose Epidural Tray |
| 04046964843884 | Single Dose Epidural Tray |
| 04046964843860 | Continuous Epidural Tray |
| 04046964843846 | Continuous Epidural Tray |
| 04046964843822 | Continuous Epidural Tray |
| 04046964843785 | PNB/Support Tray |
| 04046964814013 | Continuous Epidural Tray |
| 04046964813993 | Combined Spinal/Epidural Tray |
| 04046964809149 | Spinal Tray |
| 04046964809125 | Spinal Tray |
| 04046964809101 | Spinal Tray |
| 04046964809088 | Spinal Tray |
| 04046964809026 | Continuous Epidural Tray |
| 04046964809002 | Continuous Epidural Tray |
Trademark Results [Design Options]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
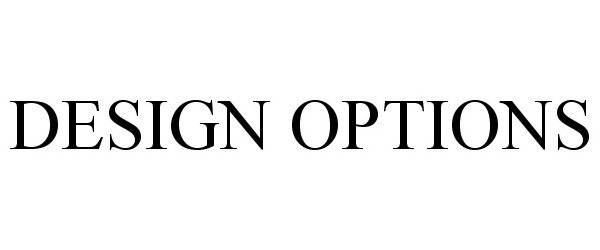 DESIGN OPTIONS 85156114 4031029 Live/Registered |
B. Braun Medical, Inc. 2010-10-19 |
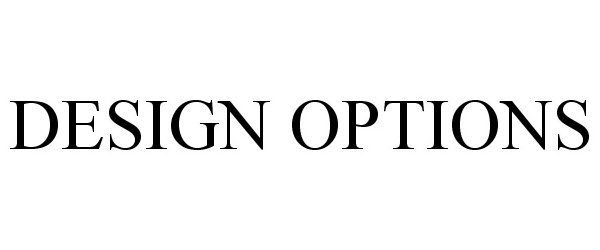 DESIGN OPTIONS 77872322 not registered Dead/Abandoned |
Mohawk Carpet Distribution, Inc. 2009-11-13 |
 DESIGN OPTIONS 76371471 2683122 Dead/Cancelled |
BYTRONICS, INC. 2002-02-19 |
 DESIGN OPTIONS 76242625 2896433 Dead/Cancelled |
B. Braun Medical, Inc. 2001-04-18 |
 DESIGN OPTIONS 75503878 2499186 Dead/Cancelled |
NORCRAFT COMPANIES, LLC 1998-06-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.