activL® ME278
GUDID 04046955848003
ACTIVL NXG SLIM TRIALING AND AUX TRAY
AESCULAP IMPLANT SYSTEMS, LLC
Instrument tray, reusable| Primary Device ID | 04046955848003 |
| NIH Device Record Key | 389acf98-7813-4915-bfe0-c5ae9e1f34c5 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | activL® |
| Version Model Number | ME278 |
| Catalog Number | ME278 |
| Company DUNS | 622600992 |
| Company Name | AESCULAP IMPLANT SYSTEMS, LLC |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04046955848003 [Primary] |
FDA Product Code
| FSM | TRAY, SURGICAL, INSTRUMENT |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
[04046955848003]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-04-22 |
| Device Publish Date | 2024-04-13 |
On-Brand Devices [activL®]
| 04046955849451 | ACTIVL NXG TRAY LID |
| 04046955849444 | ACTIVL NXG CHISEL AND IMPACTION TRAY |
| 04046955849437 | ACTIVL NXG REPOSITIONING AND AUX TRAY |
| 04046955849420 | ACTIVL NXG DISCECTOMY II TRAY |
| 04046955849413 | ACTIVL NXG DISCECTOMY I TRAY |
| 04046955848003 | ACTIVL NXG SLIM TRIALING AND AUX TRAY |
| 04046955847990 | ACTIVL NXG TRIALING TRAY |
| 04046955847174 | ACTIVL NXG INSERTION TRAY |
Trademark Results [activL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
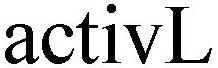 ACTIVL 79020561 3302727 Live/Registered |
Aesculap AG 2006-01-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.