MegaBeam® Endo-ENT Probe ENT-24-COH-S
GUDID 04049089300324
Ceram Optec SIA
General/multiple surgical laser system beam guide, single-use, sterile| Primary Device ID | 04049089300324 |
| NIH Device Record Key | 61811165-56b0-4b28-8170-55995c999f83 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | MegaBeam® Endo-ENT Probe |
| Version Model Number | MegaBeam® Endo-ENT Probe, 24Ga, COH-S, noID |
| Catalog Number | ENT-24-COH-S |
| Company DUNS | 506069240 |
| Company Name | Ceram Optec SIA |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04049089100320 [Primary] |
| GS1 | 04049089200327 [Package] Contains: 04049089100320 Package: Box [5 Units] In Commercial Distribution |
| GS1 | 04049089300324 [Package] Contains: 04049089200327 Package: Box [2 Units] In Commercial Distribution |
FDA Product Code
| GEX | Powered Laser Surgical Instrument |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-06-26 |
| Device Publish Date | 2017-03-07 |
On-Brand Devices [MegaBeam® Endo-ENT Probe]
| 04049089300386 | MegaBeam® Endo-ENT Probe, 24Ga, SMA-S, noID |
| 04049089300379 | MegaBeam® Endo-ENT Probe, 24Ga, SMA-H, noID |
| 04049089300362 | MegaBeam® Endo-ENT Probe, 24Ga, SMA-G, noID |
| 04049089300355 | MegaBeam® Endo-ENT Probe, 24Ga, HGM-S, noID |
| 04049089300348 | MegaBeam® Endo-ENT Probe, 24Ga, HGM-H, noID |
| 04049089300331 | MegaBeam® Endo-ENT Probe, 24Ga, HGM-G, noID |
| 04049089300324 | MegaBeam® Endo-ENT Probe, 24Ga, COH-S, noID |
| 04049089300317 | MegaBeam® Endo-ENT Probe, 24Ga, COH-H, noID |
| 04049089300300 | MegaBeam® Endo-ENT Probe, 24Ga, COH-G, noID |
Trademark Results [MegaBeam]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEGABEAM 90033344 not registered Live/Pending |
S3 Concrete Technologies, Inc. 2020-07-02 |
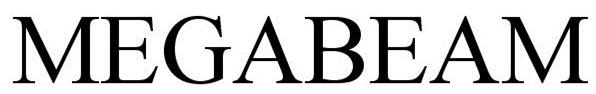 MEGABEAM 85889040 4541843 Live/Registered |
Man-D-Tec, Inc. 2013-03-28 |
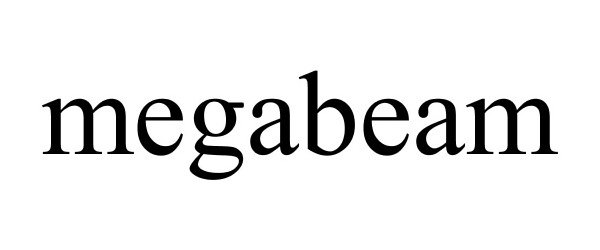 MEGABEAM 85136126 not registered Dead/Abandoned |
Locata Corporation Pty Ltd 2010-09-23 |
 MEGABEAM 76709813 4292521 Live/Registered |
ALLIED INTERNATIONAL LLC 2011-11-21 |
 MEGABEAM 76144300 not registered Dead/Abandoned |
Megabeam Media Limited 2000-10-11 |
 MEGABEAM 74667244 2029599 Dead/Cancelled |
BIOLITEC UNTERNEHMENSBETEILIGUNGS II AG 1995-04-24 |
 MEGABEAM 73613775 not registered Dead/Abandoned |
ARCTURUS, INC. 1986-08-08 |
 MEGABEAM 73099254 1062143 Dead/Expired |
SHAKESPEARE COMPANY 1976-09-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.