ACCUGLIDE® Disposable Blade 5070060
GUDID 04050555000976
Disposable blades for HANSATOME® Microkeratome.
Technolas Perfect Vision GmbH
Keratome, line-powered| Primary Device ID | 04050555000976 |
| NIH Device Record Key | 348b3b1c-d6b1-4fad-b808-abe2e6e9bc3f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | ACCUGLIDE® Disposable Blade |
| Version Model Number | 1 |
| Catalog Number | 5070060 |
| Company DUNS | 341325716 |
| Company Name | Technolas Perfect Vision GmbH |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +4989940040 |
| info@technolaspv.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04050555000976 [Primary] |
FDA Product Code
| HNO | Keratome, Ac-Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2018-12-03 |
| Device Publish Date | 2016-09-19 |
Devices Manufactured by Technolas Perfect Vision GmbH
| 04050555002086 - Patient Interface 125 Kit | 2024-03-21 |
| 04050555004202 - TECHNOLAS TENEO 317 | 2023-12-25 |
| 04050555000839 - MICROKERATOME Disposable Tubing | 2018-12-03 Provides a connection between the microkeratome head and the vacuum (or suction) pump. The function of the tubing is to provide |
| 04050555000976 - ACCUGLIDE® Disposable Blade | 2018-12-03Disposable blades for HANSATOME® Microkeratome. |
| 04050555000976 - ACCUGLIDE® Disposable Blade | 2018-12-03 Disposable blades for HANSATOME® Microkeratome. |
| 04050555000983 - Zyoptix® XP Microkeratome Disposable Blades | 2018-12-03 Disposable blades for ZYOPTIX® XP Microkeratome. |
| 04050555000990 - Patient Interface 125 Kit | 2018-12-03 Patient Interface Kit for VICTUS™ Femtosecond Laser Platform (software version 2.7 and higher). Kit consists of Patient Interf |
| 04050555002895 - VICTUS™ Femtosecond Laser Platform | 2018-12-03 The laser system is an ophthalmologic system based on femtosecond technology. It is used to treat a wide variety of ophthalmolog |
| 04050555004165 - VICTUS VERAFIT Patient Interface Kit | 2018-12-03 Patient Interface Kit for VICTUS™ Femtosecond Laser Platform (software version 2.7 and higher). Kit consists of Patient Interf |
Trademark Results [ACCUGLIDE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
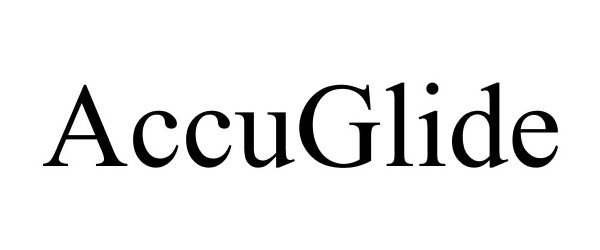 ACCUGLIDE 88602117 not registered Live/Pending |
Russell Brands, LLC 2019-09-03 |
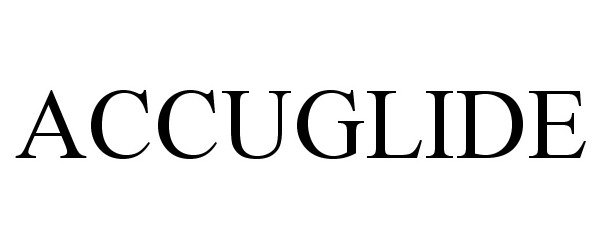 ACCUGLIDE 85737747 not registered Dead/Abandoned |
Vascular Pathways, Inc. 2012-09-25 |
 ACCUGLIDE 75704043 not registered Dead/Abandoned |
Huffy Corporation 1999-05-13 |
 ACCUGLIDE 75319255 2207687 Live/Registered |
TECHNOLAS PERFECT VISION GMBH 1997-07-03 |
 ACCUGLIDE 73604018 1425497 Live/Registered |
MINNESOTA MINING AND MANUFACTURING COMPANY 1986-06-13 |
 ACCUGLIDE 73596860 not registered Dead/Abandoned |
ALVEY, INC. 1986-05-05 |
 ACCUGLIDE 73442915 1295743 Dead/Cancelled |
Thomson Industries, Inc. 1983-09-08 |
 ACCUGLIDE 73143621 1124654 Dead/Cancelled |
ACCUTRAC LTD. 1977-10-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.