locomotion® 150/50 DE med cos30001-01va02
GUDID 04050588001506
h/p/cosmos sports & medical gmbh
Cardiopulmonary stress test treadmill| Primary Device ID | 04050588001506 |
| NIH Device Record Key | f1a8f2dc-ca60-4f35-9b4f-d89d598c631b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | locomotion® 150/50 DE med |
| Version Model Number | cos30001-01va02 |
| Catalog Number | cos30001-01va02 |
| Company DUNS | 341362614 |
| Company Name | h/p/cosmos sports & medical gmbh |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04050588001506 [Primary] |
FDA Product Code
| IOL | Treadmill, Powered |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2020-05-19 |
| Device Publish Date | 2020-01-07 |
Devices Manufactured by h/p/cosmos sports & medical gmbh
| 04050588000059 - stratos® lt med | 2020-05-19 |
| 04050588000066 - stratos® med | 2020-05-19 |
| 04050588000073 - mercury® lt med | 2020-05-19 |
| 04050588000080 - mercury® med | 2020-05-19 |
| 04050588000295 - locomotion® 150/50 E med | 2020-05-19 |
| 04050588000493 - stellar® lt med | 2020-05-19 |
| 04050588000509 - stellar® med | 2020-05-19 |
| 04050588000516 - quasar® lt med | 2020-05-19 |
Trademark Results [locomotion]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LOCOMOTION 90542890 not registered Live/Pending |
EVERI GAMES INC. 2021-02-23 |
 LOCOMOTION 85958000 not registered Dead/Abandoned |
ARTHROSURFACE INCORPORATED 2013-06-12 |
 LOCOMOTION 77252138 not registered Dead/Abandoned |
Big Train, Inc. 2007-08-10 |
 LOCOMOTION 77030282 not registered Dead/Abandoned |
EMSCO Inc. 2006-10-26 |
 LOCOMOTION 76495324 2856598 Live/Registered |
Locomotion, LLC. 2003-03-05 |
 LOCOMOTION 76470292 not registered Dead/Abandoned |
BRUGLIO, JOSEPH 2002-11-26 |
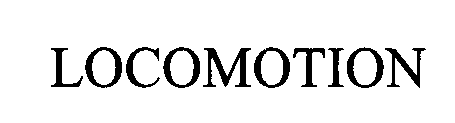 LOCOMOTION 76455239 2746894 Dead/Cancelled |
Digitrax, Inc. 2002-10-03 |
 LOCOMOTION 76110630 2584599 Dead/Cancelled |
Locomotion Channel, The 2000-08-16 |
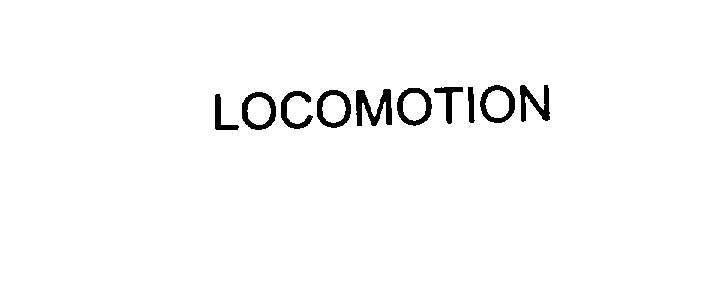 LOCOMOTION 76110628 2691433 Dead/Cancelled |
Locomotion Channel, The 2000-08-16 |
 LOCOMOTION 76106073 not registered Dead/Abandoned |
4032942 CANADA INC. 2000-08-09 |
 LOCOMOTION 76106072 not registered Dead/Abandoned |
Locomotion Channel, The 2000-08-09 |
 LOCOMOTION 76027684 not registered Dead/Abandoned |
Train Worx 2000-04-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.