DiCo
GUDID 04057217006710
Z-Medical GmbH + Co. KG
Orthopaedic surgical distractor, internal| Primary Device ID | 04057217006710 |
| NIH Device Record Key | 2f97b885-6024-4b97-baca-d28ed3629343 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | DiCo |
| Version Model Number | A06 600 |
| Company DUNS | 332068761 |
| Company Name | Z-Medical GmbH + Co. KG |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de | |
| Phone | +497462945540 |
| info@z-medical.de |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04057217006710 [Primary] |
FDA Product Code
| LXH | Orthopedic Manual Surgical Instrument |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
[04057217006710]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-04-09 |
| Device Publish Date | 2024-04-01 |
Devices Manufactured by Z-Medical GmbH + Co. KG
| 04057217003511 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 8 / 8 mm |
| 04057217003528 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 10 / 8 mm |
| 04057217003535 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 12 / 10 mm |
| 04057217003542 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 15 / 10 mm |
| 04057217003559 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 8 / 8mm 26° |
| 04057217003566 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 10 / 8mm 26° |
| 04057217003573 - Z-Staple | 2024-09-19 Z-Staple Mini Spikes 12 / 10mm 26° |
| 04057217000404 - Z-Pedicle Screw | 2024-07-15 |
Trademark Results [DiCo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
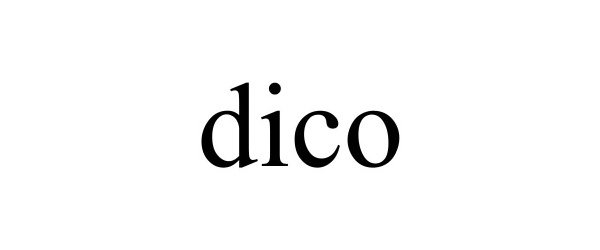 DICO 98071044 not registered Live/Pending |
LVE KOREA Inc. 2023-07-05 |
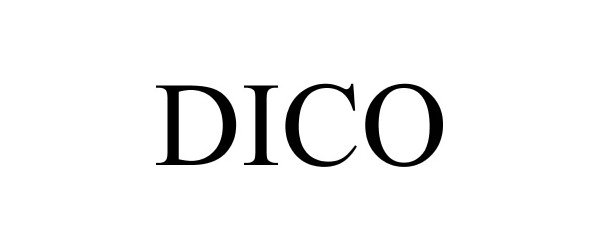 DICO 88501478 not registered Indifferent |
Chen huacong 0000-00-00 |
 DICO 79040670 3398809 Dead/Cancelled |
COR.MAR S.A.S; DI CORVARI MARINO & C. 2007-04-06 |
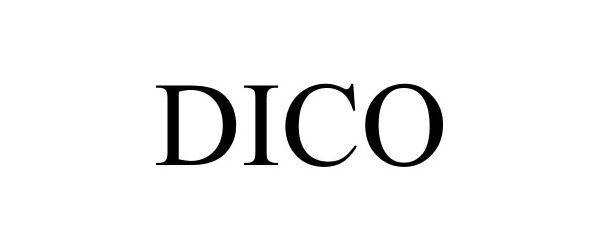 DICO 78965064 not registered Dead/Abandoned |
Muebles Dico, S.A. de C.V. 2006-08-31 |
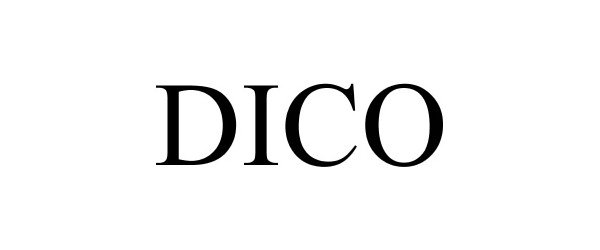 DICO 77714543 3888515 Dead/Cancelled |
MANROLAND WEB SYSTEMS GMBH 2009-04-15 |
 DICO 76160700 2667922 Dead/Cancelled |
MAN ROLAND DRUCKMASCHINEN AG 2000-11-07 |
 DICO 75566913 2295894 Live/Registered |
C. C. Dickson Co. 1998-10-08 |
 DICO 75515849 2318142 Live/Registered |
DIVINE BROTHERS COMPANY 1998-07-09 |
 DICO 74434223 1842865 Dead/Cancelled |
DICO TIRE, INC. 1993-09-10 |
 DICO 74102179 not registered Dead/Abandoned |
Muller, Josef 1990-10-01 |
 DICO 73361032 1237468 Dead/Cancelled |
Dico Sportswear Inc. 1982-05-05 |
 DICO 73357737 1306619 Dead/Cancelled |
Direct Image Corporation 1982-04-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.