ReBOSSIS ORB-0120B
GUDID 04573190050118
ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consisting of beta-tricalcium phosphate, siloxane-containin
ORTHOREBIRTH CO.,LTD.
Synthetic bone graft| Primary Device ID | 04573190050118 |
| NIH Device Record Key | 8b7c9ccf-417a-459d-a7fb-8054b7170972 |
| Commercial Distribution Discontinuation | 2018-11-11 |
| Commercial Distribution Status | Not in Commercial Distribution |
| Brand Name | ReBOSSIS |
| Version Model Number | 2.0g |
| Catalog Number | ORB-0120B |
| Company DUNS | 691435666 |
| Company Name | ORTHOREBIRTH CO.,LTD. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | 866-426-2323 |
| dblake@orthorebirthusa.com |
Device Dimensions
| Total Volume | 50 Milliliter |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dry place |
| Storage Environment Temperature | Between 0 Degrees Celsius and 25 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 04573190050118 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K142090
FDA Product Code
| MQV | Filler, Bone Void, Calcium Compound |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 4 |
| Public Version Date | 2018-11-12 |
| Device Publish Date | 2015-09-24 |
On-Brand Devices [ReBOSSIS]
| 04573190050347 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050330 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050323 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050316 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050231 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050224 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050132 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050125 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050118 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050033 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050026 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050019 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050392 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050385 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050378 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
| 04573190050309 | ReBOSSIS is a cotton-like synthetic, resorbable bone void filler. It is composite material consi |
Trademark Results [ReBOSSIS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
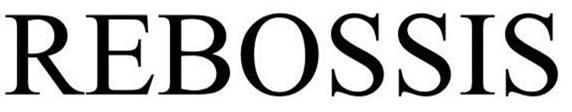 REBOSSIS 85602550 4564667 Live/Registered |
Orthorebirth Co., Ltd. 2012-04-19 |
 REBOSSIS 79232932 5694086 Live/Registered |
ORTHOREBIRTH CO., LTD. 2018-03-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.