COBE® 2991
GUDID 05020583910003
Blood Cell Processor
TERUMO BCT, INC.
Cell washer IVD| Primary Device ID | 05020583910003 |
| NIH Device Record Key | 4220e831-57ea-4c64-8ecc-18f602e6a277 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | COBE® 2991 |
| Version Model Number | 91000 |
| Company DUNS | 801679200 |
| Company Name | TERUMO BCT, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05020583910003 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K893962
FDA Product Code
| GKT | Separator, Automated, Blood Cell, Diagnostic |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2023-06-27 |
| Device Publish Date | 2023-06-19 |
On-Brand Devices [COBE® 2991 ]
| 35020583909121 | Coupler and Double Coupler Adaptor |
| 35020583909046 | Coupler and Double Coupler Adaptor |
| 35020583909015 | Triple Processing Set |
| 35020583908193 | Blood Cell Processing Set |
| 05020583909120 | Coupler and Double Coupler Adaptor |
| 05020583909045 | Coupler and Double Coupler Adaptor |
| 05020583909014 | Triple Processing Set |
| 05020583908192 | Blood Cell Processing Set |
| 05020583910003 | Blood Cell Processor |
Trademark Results [COBE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
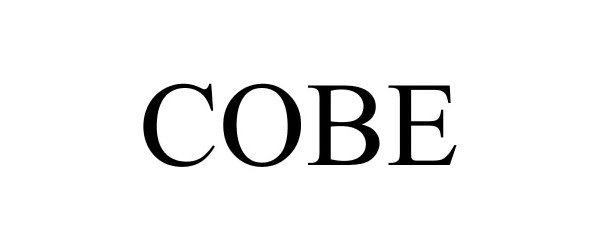 COBE 87669676 not registered Live/Pending |
Cobe ApS 2017-11-02 |
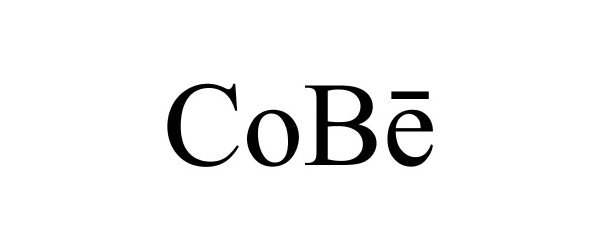 COBE 87179606 5520203 Live/Registered |
Patrick Campbell 2016-09-22 |
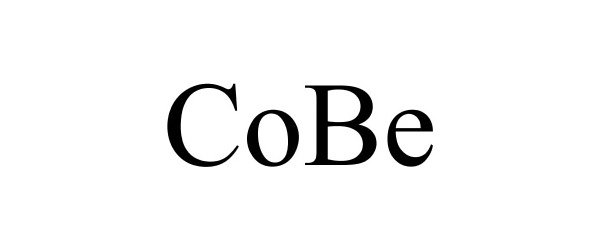 COBE 86944902 not registered Live/Pending |
Continental Automotive GmbH 2016-03-18 |
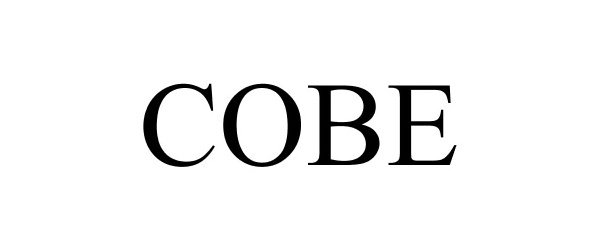 COBE 86785532 5115656 Live/Registered |
Jernudd, Judith G. 2015-10-12 |
 COBE 75908192 not registered Dead/Abandoned |
NET-TEL CORPORATION 2000-02-03 |
 COBE 75018235 2131032 Live/Registered |
TERUMO BCT, INC. 1995-11-13 |
 COBE 74599256 1974362 Dead/Cancelled |
GAMBRO, INC. 1994-11-15 |
 COBE 73725669 1515251 Live/Registered |
COBE LABORATORIES, INC. 1988-05-02 |
 COBE 73181319 1128505 Dead/Cancelled |
COBE LABORATORIES, INC. 1978-08-07 |
 COBE 72403054 0976436 Dead/Expired |
COBE LABORATORIES, INC. 1971-09-20 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.