I-GEL® PLUS ANAESTHESIA, SUPRAGLOTTIC AIRWAY, SIZE 3, SMALL ADULT, 30-60KG 8903000
GUDID 05030267166367
I-GEL® PLUS ANAESTHESIA, SUPRAGLOTTIC AIRWAY, SIZE 3, SMALL ADULT, 30-60KG
Intersurgical Incorporated
Laryngeal mask airway, single-use| Primary Device ID | 05030267166367 |
| NIH Device Record Key | 7ba1a7d8-e609-4346-88d2-e07a7e0e9b4d |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | I-GEL® PLUS ANAESTHESIA, SUPRAGLOTTIC AIRWAY, SIZE 3, SMALL ADULT, 30-60KG |
| Version Model Number | 8903000 |
| Catalog Number | 8903000 |
| Company DUNS | 785952607 |
| Company Name | Intersurgical Incorporated |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | MR Safe |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | true |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | true |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com | |
| Phone | +3154512900 |
| support@intersurgicalinc.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05030267165759 [Primary] |
| GS1 | 05030267166367 [Package] Contains: 05030267165759 Package: [10 Units] In Commercial Distribution |
FDA Product Code
| CAE | Airway, Oropharyngeal, Anesthesiology |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2025-04-22 |
| Device Publish Date | 2025-04-14 |
Devices Manufactured by Intersurgical Incorporated
Trademark Results [I-GEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
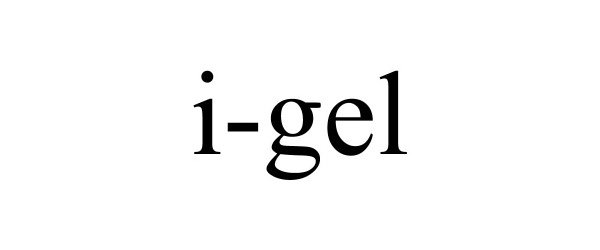 I-GEL 90873056 not registered Live/Pending |
Murray & Pool Enterprises Ltd. 2021-08-09 |
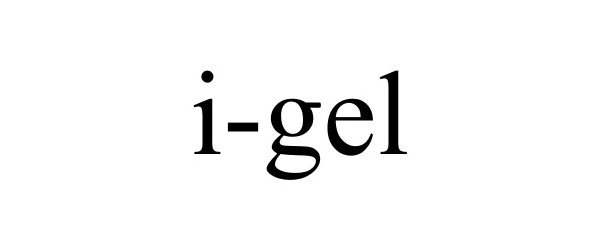 I-GEL 85727044 not registered Dead/Abandoned |
Murray & Pool Enterprises Ltd. 2012-09-12 |
 I-GEL 79297843 not registered Live/Pending |
Murray & Pool Enterprises Ltd. 2020-09-21 |
 I-GEL 79276609 not registered Live/Pending |
Intersurgical Ltd 2019-07-23 |
 I-GEL 78298625 2993205 Dead/Cancelled |
IRWIN INDUSTRIAL TOOL COMPANY 2003-09-10 |
 I-GEL 78190149 2917197 Dead/Cancelled |
MEGA BRANDS INTERNATIONAL, LUXEMBOURG, ZUG BRANCH 2002-12-02 |
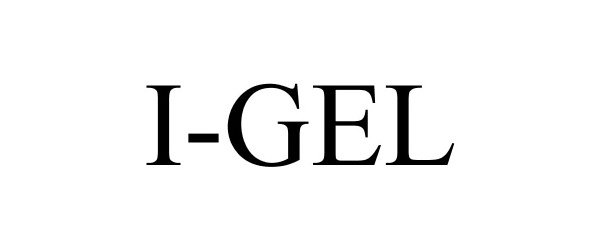 I-GEL 77263559 3532039 Live/Registered |
Intersurgical Ltd 2007-08-24 |
 I-GEL 76200897 not registered Dead/Abandoned |
Rose Art Industries, Inc. 2001-01-29 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.