Stability HS1315
GUDID 05051693300729
Flanged Uncemented Hip Stem - Size 4 - 167mm long (Cobalt Chrome) 12/14 Taper
IMPLANTS INTERNATIONAL LTD
Coated hip femur prosthesis, modular| Primary Device ID | 05051693300729 |
| NIH Device Record Key | 6abaefcd-ce42-486a-b349-6b5269828a9e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Stability |
| Version Model Number | Flanged Uncemented Hip Stem - Size 4 |
| Catalog Number | HS1315 |
| Company DUNS | 346627222 |
| Company Name | IMPLANTS INTERNATIONAL LTD |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
| Length | 167 Millimeter |
Operating and Storage Conditions
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
| Special Storage Condition, Specify | Between 0 and 0 *Room temperature and Dry |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05051693300729 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K922613
FDA Product Code
| MEH | Prosthesis, Hip, Semi-Constrained, Uncemented, Metal/Polymer, Non-Porous, Calicum-Phosphate |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 2 |
| Public Version Date | 2018-03-29 |
| Device Publish Date | 2016-07-31 |
On-Brand Devices [Stability]
| 05051693300743 | Flanged Uncemented Hip Stem - Size 6 - 174mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300736 | Flanged Uncemented Hip Stem - Size 5 - 170mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300729 | Flanged Uncemented Hip Stem - Size 4 - 167mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300712 | Flanged Uncemented Hip Stem - Size 3 - 163mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300705 | Flanged Uncemented Hip Stem - Size 2 - 157mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300699 | Flanged Uncemented Hip Stem - Size 1 - 155mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300682 | Standard Cemented Hip Stem - Size 6 - 174mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300675 | Standard Cemented Hip Stem - Size 5 - 170mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300668 | Standard Cemented Hip Stem - Size 4 - 167mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300651 | Standard Cemented Hip Stem - Size 3 - 163mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300644 | Standard Cemented Hip Stem - Size 2 - 158mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300637 | Standard Cemented Hip Stem - Size 1 - 155mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300620 | Flanged Cemented Hip Stem - Size 6 - 174mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300613 | Flanged Cemented Hip Stem - Size 5 - 170mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300606 | Flanged Cemented Hip Stem - Size 4 - 167mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300590 | Flanged Cemented Hip Stem - Size 3 - 163mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300583 | Flanged Cemented Hip Stem - Size 2 - 158mm long (Cobalt Chrome) 12/14 Taper |
| 05051693300576 | Flanged Cemented Hip Stem - Size 1 - 155mm long (Cobalt Chrome)12/14 Taper |
Trademark Results [Stability]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 STABILITY 98545750 not registered Live/Pending |
Excel LLC 2024-05-11 |
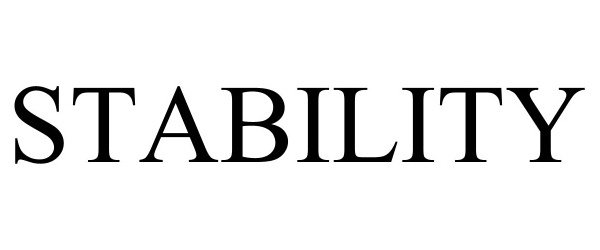 STABILITY 97861298 not registered Live/Pending |
Stability Solutions, Inc. 2023-03-28 |
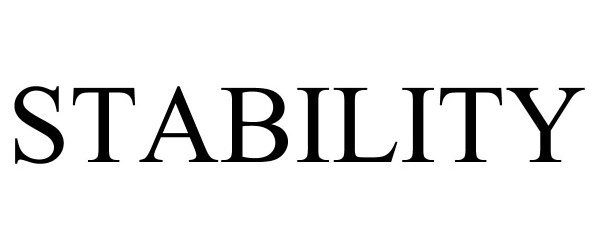 STABILITY 90852563 not registered Live/Pending |
Zibrio, Inc. 2021-07-28 |
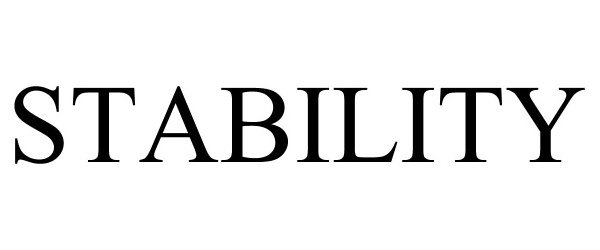 STABILITY 90617918 not registered Live/Pending |
BCBSM, Inc. 2021-04-01 |
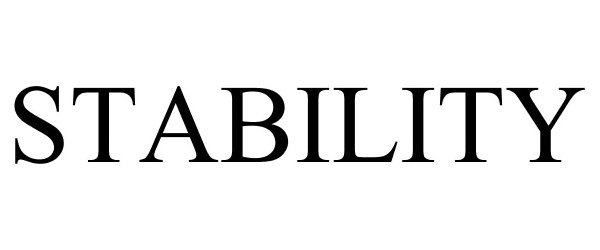 STABILITY 88524849 not registered Live/Pending |
Wilbur-Ellis Company LLC 2019-07-19 |
 STABILITY 86784830 4946289 Live/Registered |
Excel Equine, LLC 2015-10-12 |
 STABILITY 86007585 not registered Dead/Abandoned |
Global Energy Innovations, Inc. 2013-07-11 |
 STABILITY 78499648 3053903 Dead/Cancelled |
Kelly, Cory 2004-10-14 |
 STABILITY 78363932 not registered Dead/Abandoned |
Reckitt Benckiser Inc. 2004-02-06 |
 STABILITY 77582077 3615759 Live/Registered |
Seachem Laboratories, Inc. 2008-09-30 |
 STABILITY 77558776 3703101 Dead/Cancelled |
Glaxo Group Limited 2008-08-29 |
 STABILITY 74332819 1783824 Dead/Cancelled |
Jensen Industries Inc. 1992-11-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.