Optilite® Low Level Total Protein Reagent
GUDID 05051700020558
THE BINDING SITE GROUP LIMITED
Total protein IVD, reagent| Primary Device ID | 05051700020558 |
| NIH Device Record Key | a2d8d4e0-108c-42fa-86bf-5a19ec324d1b |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Optilite® Low Level Total Protein Reagent |
| Version Model Number | NK061.L.OPT.A |
| Company DUNS | 347045614 |
| Company Name | THE BINDING SITE GROUP LIMITED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | true |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com | |
| Phone | +4401214569500 |
| info@bindingsite.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
| Storage Environment Temperature | Between 2 Degrees Celsius and 8 Degrees Celsius |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05051700020558 [Primary] |
FDA Product Code
| DFI | Total Spinal-Fluid, Antigen, Antiserum, Control |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-11-07 |
| Device Publish Date | 2024-10-30 |
Devices Manufactured by THE BINDING SITE GROUP LIMITED
Trademark Results [Optilite]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OPTILITE 90518138 not registered Live/Pending |
Kulicke and Soffa Industries, Inc. 2021-02-08 |
 OPTILITE 90331654 not registered Live/Pending |
LW 602, LLC 2020-11-20 |
 OPTILITE 87648610 5474976 Live/Registered |
The Binding Site, Ltd. 2017-10-17 |
 OPTILITE 87262258 5419348 Live/Registered |
CWI, Inc. 2016-12-08 |
 OPTILITE 87050190 not registered Dead/Abandoned |
Xintec Corporation 2016-05-25 |
 OPTILITE 85868285 4431718 Live/Registered |
The Binding Site Group, Ltd. 2013-03-06 |
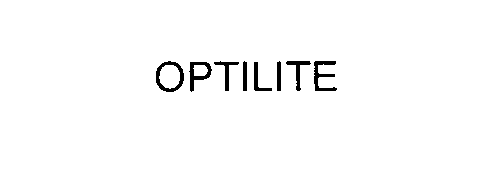 OPTILITE 75546012 2491092 Dead/Cancelled |
BELDEN TECHNOLOGIES, INC. 1998-09-01 |
 OPTILITE 74153285 1719799 Dead/Cancelled |
DEMETRON RESEARCH CORPORATION 1991-04-02 |
 OPTILITE 73838163 1642179 Dead/Cancelled |
DOW CORNING CORPORATION 1989-11-13 |
 OPTILITE 73828674 1608257 Dead/Cancelled |
BAYER AKTIENGESELLSCHAFT 1989-10-02 |
 OPTILITE 72008293 0637134 Live/Registered |
UNITED STATES SAFETY SERVICE CO. 1956-05-14 |
 OPTILITE 71498447 0509544 Dead/Expired |
A. F. PARMELLE 1948-03-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.