Biogel® Skinsense® Indicator® System
GUDID 05060097937738
Biogel Skinsense Underglove 6.5x50
Mölnlycke Health Care AB
Polychloroprene surgical glove, non-powdered| Primary Device ID | 05060097937738 |
| NIH Device Record Key | 92a6b136-475d-4a08-9387-2fab1b779f79 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Biogel® Skinsense® Indicator® System |
| Version Model Number | 40665 |
| Company DUNS | 631770658 |
| Company Name | Mölnlycke Health Care AB |
| Device Count | 1 |
| DM Exempt | true |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | false |
| OTC Over-The-Counter | false |
Customer Support Contacts
Device Dimensions
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
| Device Size Text, specify | 0 |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05060097937578 [Primary] |
| GS1 | 05060097937653 [Package] Contains: 05060097937578 Package: PACK_OR_INNER_PACK [50 Units] In Commercial Distribution |
| GS1 | 05060097937738 [Package] Contains: 05060097937653 Package: CASE [4 Units] In Commercial Distribution |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K000421
FDA Product Code
| KGO | Surgeon's gloves |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2022-10-31 |
| Device Publish Date | 2022-10-21 |
On-Brand Devices [Biogel® Skinsense® Indicator® System]
| 05060097937707 | Biogel Skinsense Underglove 9.0x40 |
| 05060097937776 | Biogel Skinsense Underglove 8.5x50 |
| 05060097937684 | Biogel Skinsense Underglove 8.0x50 |
| 05060097937752 | Biogel Skinsense Underglove 7.5x50 |
| 05060097937660 | Biogel Skinsense Underglove 7.0x50 |
| 05060097937738 | Biogel Skinsense Underglove 6.5x50 |
| 05060097937646 | Biogel Skinsense Underglove 6.0x50 |
| 05060097937639 | Biogel Skinsense Underglove 5.5x50 |
Trademark Results [Biogel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
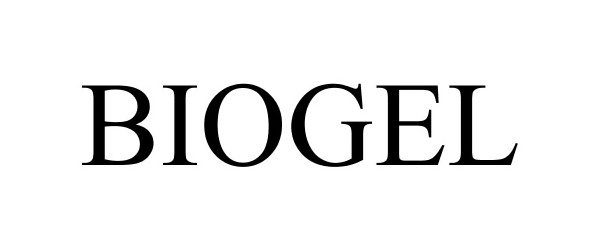 BIOGEL 87684161 not registered Dead/Abandoned |
PHOENIX Chemical, Inc. 2017-11-14 |
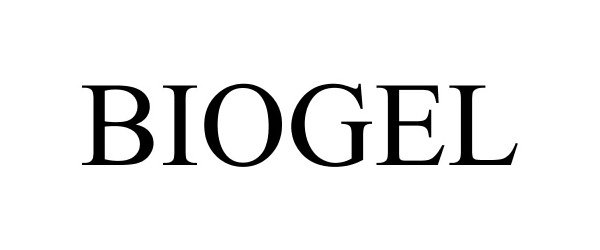 BIOGEL 86217294 4730182 Live/Registered |
Phoenix Chemical Inc. 2014-03-11 |
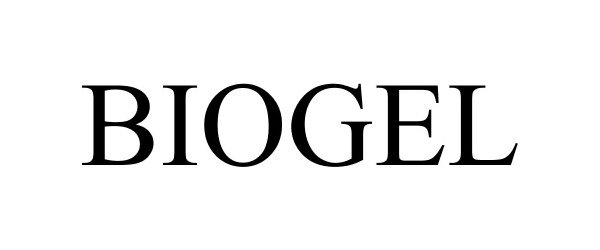 BIOGEL 85595270 4389014 Live/Registered |
Louis Garneau Sports Inc. 2012-04-11 |
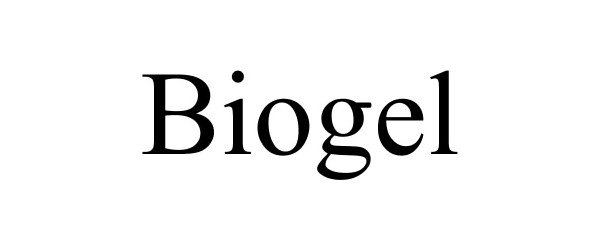 BIOGEL 85481846 4315606 Dead/Cancelled |
Rothbard, Michael 2011-11-28 |
 BIOGEL 78473714 not registered Dead/Abandoned |
IVREA Inc. 2004-08-25 |
 BIOGEL 78135510 3013748 Dead/Cancelled |
REGENT MEDICAL LIMITED 2002-06-13 |
 BIOGEL 77858173 not registered Dead/Abandoned |
Meynard Designs, Inc. 2009-10-27 |
 BIOGEL 77565224 4523726 Live/Registered |
Summers, Adam N 2008-09-09 |
 BIOGEL 77157241 not registered Dead/Abandoned |
Lincoln L. Neal 2007-04-16 |
 BIOGEL 76639768 not registered Dead/Abandoned |
Electromed, Inc. 2005-05-31 |
 BIOGEL 76626918 3254405 Dead/Cancelled |
Phoenix Chemical Inc. 2005-01-04 |
 BIOGEL 75133602 2128295 Live/Registered |
REGENT MEDICAL LIMITED 1996-07-12 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.