Apex™
GUDID 05060191071215
Apex
ELEKTA LIMITED
Automatic-aperture-control accelerator system collimator| Primary Device ID | 05060191071215 |
| NIH Device Record Key | 90745627-940c-448b-8e16-80e4699df47f |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Apex™ |
| Version Model Number | 1526774 |
| Company DUNS | 525557559 |
| Company Name | ELEKTA LIMITED |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | false |
| Lot Batch | false |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | false |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com | |
| Phone | +1(855)693-5358 |
| systems.support.na@elekta.com |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05060191071215 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K111676
FDA Product Code
| IXI | Block, beam-shaping, radiation therapy |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | false |
Device Entry Metadata
| Public Version Status | Update |
| Device Record Status | Published |
| Public Version Number | 3 |
| Public Version Date | 2018-07-06 |
| Device Publish Date | 2016-09-24 |
Devices Manufactured by ELEKTA LIMITED
| 05060191071468 - Elekta Unity | 2021-12-08 CT OVERLAY |
| 05060191071482 - Elekta Unity | 2021-12-08 Indexing Bar |
| 05060191071499 - Elekta Unity | 2021-12-08 Indexing Bars |
| 05060191071598 - Elekta Unity | 2021-12-08 Elekta Unity |
| 05060191071345 - Integrity™ | 2020-01-20 Integrity |
| 05060191071420 - Elekta Unity | 2019-05-08 INDEXING BAR |
| 05060191071437 - Elekta Unity | 2019-05-08 INDEXING BARS |
| 05060191071321 - Elekta Unity | 2019-02-25 Elekta Unity |
Trademark Results [Apex]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 APEX 98811987 not registered Live/Pending |
AFTERMARKET PERFORMANCE EXPRESS 2024-10-21 |
 APEX 98805060 not registered Live/Pending |
Dongguan Luxshare Technology Co., Ltd. 2024-10-16 |
 APEX 98733536 not registered Live/Pending |
Apex natural nutrition inc. 2024-09-04 |
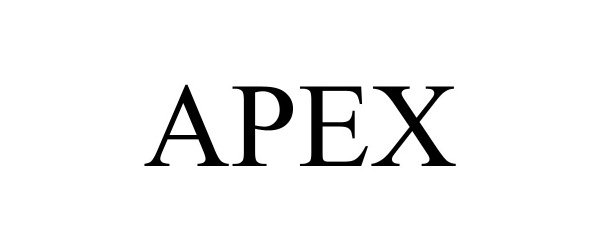 APEX 98674096 not registered Live/Pending |
Medtronic Xomed, Inc. 2024-07-30 |
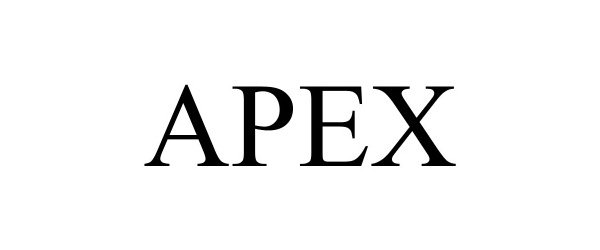 APEX 98673954 not registered Live/Pending |
Covert Instruments LLC 2024-07-30 |
 APEX 98668271 not registered Live/Pending |
Smith-Root, Inc. 2024-07-26 |
 APEX 98662611 not registered Live/Pending |
FRC Global Inc. 2024-07-23 |
 APEX 98644654 not registered Live/Pending |
Blume, Phillip James 2024-07-11 |
 APEX 98636442 not registered Live/Pending |
Catalina Carpet Mills Inc. 2024-07-08 |
 APEX 98616917 not registered Live/Pending |
Lander Hospitality Holdings LLC 2024-06-25 |
 APEX 98616917 not registered Live/Pending |
Hines, Victor Nunzio 2024-06-25 |
 APEX 98616917 not registered Live/Pending |
Comes, Bryan 2024-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.