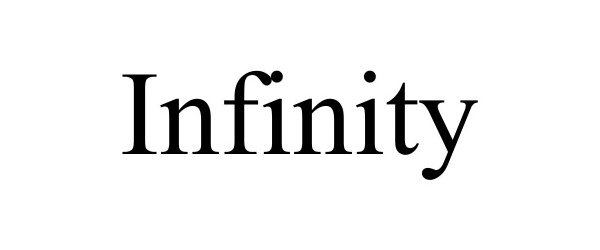Infinity™ 6660
GUDID 05415067030016
Implantable Pulse Generator
ST. JUDE MEDICAL, INC.
Deep brain electrical stimulation system| Primary Device ID | 05415067030016 |
| NIH Device Record Key | dbd28cee-792a-4bc8-9f43-6a458ee75653 |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | Infinity™ |
| Version Model Number | 5 |
| Catalog Number | 6660 |
| Company DUNS | 149818952 |
| Company Name | ST. JUDE MEDICAL, INC. |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | MR Conditional |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | true |
| Manufacturing Date | true |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Customer Support Contacts
| Phone | +1(855)478-5833 |
| customerservice@sjm.com |
Operating and Storage Conditions
| Storage Environment Temperature | Between -20 Degrees Celsius and 60 Degrees Celsius |
| Storage Environment Temperature | Between -4 Degrees Fahrenheit and 140 Degrees Fahrenheit |
| Special Storage Condition, Specify | Between 0 and 0 *Store in a dry place |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05415067030016 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Approval: P140009
FDA Product Code
| MHY | STIMULATOR, ELECTRICAL, IMPLANTED, FOR PARKINSONIAN TREMOR |
Sterilization
| Steralize Prior To Use | false |
| Device Is Sterile | true |
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2018-09-24 |
| Device Publish Date | 2018-08-24 |
On-Brand Devices [Infinity™]
| 05415067030023 | Implantable Pulse Generator |
| 05415067030016 | Implantable Pulse Generator |
| 05415067023988 | Patient Manual and Magnet |
| 05415067020277 | Implantable Pulse Generator |
| 05415067020253 | Implantable Pulse Generator |
| 05415067020246 | Implantable Pulse Generator |
Trademark Results [Infinity]
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.