AtlasPlan SHC-IN05-00-01
GUDID 05420060367120
Guide and Bone model for Reverse Shoulder Arthroplasty
Materialise NV
Custom-made organ/bone anatomy model/surgical guide kit| Primary Device ID | 05420060367120 |
| NIH Device Record Key | a134d931-bf7f-4b30-b3a1-4bb5cf31ec6e |
| Commercial Distribution Status | In Commercial Distribution |
| Brand Name | AtlasPlan |
| Version Model Number | SHC-IN05-00-01 |
| Catalog Number | SHC-IN05-00-01 |
| Company DUNS | 373139427 |
| Company Name | Materialise NV |
| Device Count | 1 |
| DM Exempt | false |
| Pre-market Exempt | false |
| MRI Safety Status | Labeling does not contain MRI Safety Information |
| Human Cell/Tissue Product | false |
| Device Kit | false |
| Device Combination Product | false |
| Single Use | true |
| Lot Batch | true |
| Serial Number | false |
| Manufacturing Date | false |
| Expiration Date | true |
| Donation Id Number | false |
| Contains Natural Rubber Latex | false |
| Labeled No Natural Rubber Latex | false |
| RX Perscription | true |
| OTC Over-The-Counter | false |
Device Identifiers
| Device Issuing Agency | Device ID |
|---|---|
| GS1 | 05420060367120 [Primary] |
FDA Pre-market Approvals/Notifications & deNovo
- Premarket Notification: K233408
FDA Product Code
| KWS | Prosthesis, Shoulder, Semi-Constrained, Metal/Polymer Cemented |
Sterilization
| Steralize Prior To Use | true |
| Device Is Sterile | false |
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
[05420060367120]
Moist Heat or Steam Sterilization
Device Entry Metadata
| Public Version Status | New |
| Device Record Status | Published |
| Public Version Number | 1 |
| Public Version Date | 2024-07-29 |
| Device Publish Date | 2024-07-19 |
On-Brand Devices [AtlasPlan]
| 05420060367038 | Guide and Bone model for Total Shoulder Arthroplasty |
| 05420060367007 | Guide and Bone model for Reverse Shoulder Arthroplasty |
| 05420060367106 | Bone model for Total Shoulder Arthroplasty Aetos |
| 05420060367090 | Guide for Total Shoulder Arthroplasty Aetos |
| 05420060367083 | Guide and Bone model for Total Shoulder Arthroplasty Aetos |
| 05420060367076 | Guide for Reverse Shoulder Arthroplasty Aetos |
| 05420060367069 | Guide and Bone model for Reverse Shoulder Arthroplasty Aetos |
| 05420060367052 | Bone model for Reverse Shoulder Arthroplasty Aetos |
| 05420060367120 | Guide and Bone model for Reverse Shoulder Arthroplasty |
Trademark Results [AtlasPlan]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ATLASPLAN 79353062 not registered Live/Pending |
CERAMICHE ATLAS CONCORDE S.P.A. 2022-06-27 |
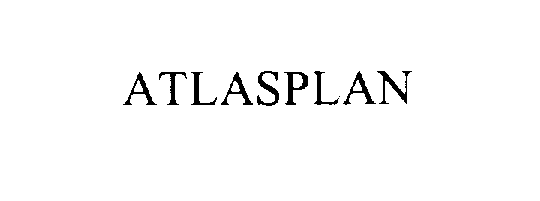 ATLASPLAN 76154122 2552201 Dead/Cancelled |
INTEGRA RADIONICS, INC. 2000-10-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.